Brand Name
Idacio
Generic Name
Adalimumab-Aacf
View Brand Information FDA approval date: July 01, 2023
Form: Kit
What is Idacio (Adalimumab-Aacf)?
IDACIO is a tumor necrosis factor blocker indicated for: Rheumatoid Arthritis .
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
IDACIO (adalimumab-aacf)
WARNING: SERIOUS INFECTIONS and MALIGNANCY
SERIOUS INFECTIONS
Patients treated with adalimumab products including IDACIO are at increased risk for developing serious infections that may lead to hospitalization or death
Discontinue IDACIO if a patient develops a serious infection or sepsis. Reported infections include:
- Active tuberculosis (TB), including reactivation of latent TB. Patients with TB have frequently presented with disseminated or extrapulmonary disease. Test patients for latent TB before IDACIO use and during therapy. Initiate treatment for latent TB prior to IDACIO use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti- fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria.
Carefully consider the risks and benefits of treatment with IDACIO prior to initiating therapy in patients with chronic or recurrent infection.
Monitor patients closely for the development of signs and symptoms of infection during and after treatment with IDACIO, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers including adalimumab products
1DOSAGE FORMS AND STRENGTHS
IDACIO is a clear and colorless to pale yellow solution available as:
- Pen (IDACIO Pen)
- Injection: 40 mg/0.8 mL in a single-dose pen.
- Prefilled Syringe
- Injection: 40 mg/0.8 mL in a single-dose prefilled glass syringe.
- Single-Dose Institutional Use Vial Kit
- Injection: 40 mg/0.8 mL in a single-dose, glass vial kit for institutional use only.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Infections
- Malignancies
- Hypersensitivity Reactions
- Hepatitis B Virus Reactivation
- Neurologic Reactions
- Hematological Reactions
- Heart Failure
- Autoimmunity
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reaction with adalimumab was injection site reactions. In placebo- controlled trials, 20% of patients treated with adalimumab developed injection site reactions (erythema and/or itching, hemorrhage, pain or swelling), compared to 14% of patients receiving placebo. Most injection site reactions were described as mild and generally did not necessitate drug discontinuation.
The proportion of patients who discontinued treatment due to adverse reactions during the double-blind, placebo-controlled portion of studies in patients with RA (i.e., Studies RA-I, RA‑ II, RA-III and RA-IV) was 7% for patients taking adalimumab and 4% for placebo-treated patients. The most common adverse reactions leading to discontinuation of adalimumab in these RA studies were clinical flare reaction (0.7%), rash (0.3%) and pneumonia (0.3%).
Infections
In the controlled portions of the 39 global adalimumab clinical trials in adult patients with RA, PsA, AS, CD, UC, Ps, HS and UV, the rate of serious infections was 4.3 per 100 patient-years in 7973 adalimumab-treated patients versus a rate of 2.9 per 100 patient-years in 4848 control-treated patients. Serious infections observed included pneumonia, septic arthritis, prosthetic and post- surgical infections, erysipelas, cellulitis, diverticulitis, and pyelonephritis
Tuberculosis and Opportunistic Infections
In 52 global controlled and uncontrolled clinical trials in RA, PsA, AS, CD, UC, Ps, HS, and UV that included 24,605 adalimumab-treated patients, the rate of reported active tuberculosis was 0.20 per 100 patient-years and the rate of positive PPD conversion was 0.09 per 100 patient-years. In a subgroup of 10,113 U.S. and Canadian adalimumab-treated patients, the rate of reported active TB was 0.05 per 100 patient-years and the rate of positive PPD conversion was 0.07 per 100 patient-years. These trials included reports of miliary, lymphatic, peritoneal, and pulmonary TB. Most of the TB cases occurred within the first eight months after initiation of therapy and may reflect recrudescence of latent disease. In these global clinical trials, cases of serious opportunistic infections have been reported at an overall rate of 0.05 per 100 patient-years. Some cases of serious opportunistic infections and TB have been fatal
Autoantibodies
In the rheumatoid arthritis controlled trials, 12% of patients treated with adalimumab and 7% of placebo-treated patients that had negative baseline ANA titers developed positive titers at week 24. Two patients out of 3046 treated with adalimumab developed clinical signs suggestive of new- onset lupus-like syndrome. The patients improved following discontinuation of therapy. No patients developed lupus nephritis or central nervous system symptoms. The impact of long-term treatment with adalimumab products on the development of autoimmune diseases is unknown.
Liver Enzyme Elevations
There have been reports of severe hepatic reactions including acute liver failure in patients receiving TNF-blockers. In controlled Phase 3 trials of adalimumab (40 mg SC every other week) in patients with RA, PsA, and AS with control period duration ranging from 4 to 104 weeks, ALT elevations ≥ 3 x ULN occurred in 3.5% of adalimumab-treated patients and 1.5% of control- treated patients. Since many of these patients in these trials were also taking medications that cause liver enzyme elevations (e.g., NSAIDS, MTX), the relationship between adalimumab and the liver enzyme elevations is not clear. In a controlled Phase 3 trial of adalimumab in patients with polyarticular JIA who were 4 to 17 years, ALT elevations ≥ 3 x ULN occurred in 4.4% of adalimumab-treated patients and 1.5% of control-treated patients (ALT more common than AST); liver enzyme test elevations were more frequent among those treated with the combination of adalimumab and MTX than those treated with adalimumab alone. In general, these elevations did not lead to discontinuation of adalimumab treatment. No ALT elevations ≥ 3 x ULN occurred in the open-label study of adalimumab in patients with polyarticular JIA who were 2 to <4 years.
In controlled Phase 3 trials of adalimumab (initial doses of 160 mg and 80 mg, or 80 mg and 40 mg on Days 1 and 15, respectively, followed by 40 mg every other week) in adult patients with Crohn’s Disease with a control period duration ranging from 4 to 52 weeks, ALT elevations ≥ 3 x ULN occurred in 0.9% of adalimumab-treated patients and 0.9% of control-treated patients. In the Phase 3 trial of adalimumab in pediatric patients with Crohn’s disease which evaluated efficacy and safety of two body weight based maintenance dose regimens following body weight based induction therapy up to 52 weeks of treatment, ALT elevations ≥ 3 x ULN occurred in 2.6% (5/192) of patients, of whom 4 were receiving concomitant immunosuppressants at baseline; none of these patients discontinued due to abnormalities in ALT tests. In controlled Phase 3 trials of adalimumab (initial doses of 160 mg and 80 mg on Days 1 and 15 respectively, followed by 40 mg every other week) in adult patients with UC with control period duration ranging from 1 to 52 weeks, ALT elevations ≥3 x ULN occurred in 1.5% of adalimumab-treated patients and 1.0% of control-treated patients. In controlled Phase 3 trials of adalimumab (initial dose of 80 mg then 40 mg every other week) in patients with Ps with control period duration ranging from 12 to 24 weeks, ALT elevations ≥ 3 x ULN occurred in 1.8% of adalimumab-treated patients and 1.8% of control-treated patients. In controlled trials of adalimumab (initial doses of 160 mg at Week 0 and 80 mg at Week 2, followed by 40 mg every week starting at Week 4), in subjects with HS with a control period duration ranging from 12 to 16 weeks, ALT elevations ≥ 3 x ULN occurred in 0.3% of adalimumab-treated subjects and 0.6% of control-treated subjects. In controlled trials of adalimumab (initial doses of 80 mg at Week 0 followed by 40 mg every other week starting at Week 1) in adult patients with uveitis with an exposure of 165.4 PYs and 119.8 PYs in adalimumab-treated and control-treated patients, respectively, ALT elevations ≥ 3 x ULN occurred in 2.4% of adalimumab-treated patients and 2.4% of control-treated patients.
Other Adverse Reactions
Rheumatoid Arthritis Clinical Studies
The data described below reflect exposure to adalimumab in 2468 patients, including 2073 exposed for 6 months, 1497 exposed for greater than one year and 1380 in adequate and well-controlled studies (Studies RA-I, RA-II, RA-III, and RA-IV). Adalimumab was studied primarily in placebo- controlled trials and in long-term follow up studies for up to 36 months duration. The population had a mean age of 54 years, 77% were female, 91% were Caucasian and had moderately to severely active rheumatoid arthritis. Most patients received 40 mg adalimumab every other week
Table 1 summarizes reactions reported at a rate of at least 5% in patients treated with adalimumab 40 mg every other week compared to placebo and with an incidence higher than placebo. In Study RA-III, the types and frequencies of adverse reactions in the second year open-label extension were similar to those observed in the one-year double-blind portion.
Less Common Adverse Reactions in Rheumatoid Arthritis Clinical Studies
Other infrequent serious adverse reactions that do not appear in the Warnings and Precautions or Adverse Reaction sections that occurred at an incidence of less than 5% in adalimumab-treated patients in RA studies were:
Body As A Whole: Pain in extremity, pelvic pain, surgery, thorax pain
Cardiovascular System: Arrhythmia, atrial fibrillation, chest pain, coronary artery disorder, heart arrest, hypertensive encephalopathy, myocardial infarct, palpitation, pericardial effusion, pericarditis, syncope, tachycardia
Digestive System: Cholecystitis, cholelithiasis, esophagitis, gastroenteritis, gastrointestinal hemorrhage, hepatic necrosis, vomiting
Endocrine System: Parathyroid disorder
Hemic And Lymphatic System: Agranulocytosis, polycythemia
Metabolic And Nutritional Disorders: Dehydration, healing abnormal, ketosis, paraproteinemia, peripheral edema
Musculo-Skeletal System: Arthritis, bone disorder, bone fracture (not spontaneous), bone necrosis, joint disorder, muscle cramps, myasthenia, pyogenic arthritis, synovitis, tendon disorder
Neoplasia: Adenoma
Nervous System: Confusion, paresthesia, subdural hematoma, tremor
Respiratory System: Asthma, bronchospasm, dyspnea, lung function decreased, pleural effusion
Special Senses: Cataract
Thrombosis: Thrombosis leg
Urogenital System: Cystitis, kidney calculus, menstrual disorder
Juvenile Idiopathic Arthritis Clinical Studies
In general, the adverse reactions in the adalimumab-treated patients in the polyarticular juvenile idiopathic arthritis (JIA) trials (Studies JIA-I and JIA-II) [
In Study JIA-I, adalimumab was studied in 171 patients who were 4 to 17 years of age, with polyarticular JIA. Severe adverse reactions reported in the study included neutropenia, streptococcal pharyngitis, increased aminotransferases, herpes zoster, myositis, metrorrhagia, and appendicitis. Serious infections were observed in 4% of patients within approximately 2 years of initiation of treatment with adalimumab and included cases of herpes simplex, pneumonia, urinary tract infection, pharyngitis, and herpes zoster.
In Study JIA-I, 45% of patients experienced an infection while receiving adalimumab with or without concomitant MTX in the first 16 weeks of treatment. The types of infections reported in adalimumab-treated patients were generally similar to those commonly seen in polyarticular JIA patients who are not treated with TNF blockers. Upon initiation of treatment, the most common adverse reactions occurring in this patient population treated with adalimumab were injection site pain and injection site reaction (19% and 16%, respectively). A less commonly reported adverse event in patients receiving adalimumab was granuloma annulare which did not lead to discontinuation of adalimumab treatment.
In the first 48 weeks of treatment in Study JIA-I, non-serious hypersensitivity reactions were seen in approximately 6% of patients and included primarily localized allergic hypersensitivity reactions and allergic rash.
In Study JIA-I, 10% of patients treated with adalimumab who had negative baseline anti-dsDNA antibodies developed positive titers after 48 weeks of treatment. No patient developed clinical signs of autoimmunity during the clinical trial.
Approximately 15% of patients treated with adalimumab developed mild-to-moderate elevations of creatine phosphokinase (CPK) in Study JIA-I. Elevations exceeding 5 times the upper limit of normal were observed in several patients. CPK concentrations decreased or returned to normal in all patients. Most patients were able to continue adalimumab without interruption.
In Study JIA-II, adalimumab was studied in 32 patients who were 2 to <4 years of age or 4 years of age and older weighing <15 kg with polyarticular JIA. The safety profile for this patient population was similar to the safety profile seen in patients 4 to 17 years of age with polyarticular JIA.
In Study JIA-II, 78% of patients experienced an infection while receiving adalimumab. These included nasopharyngitis, bronchitis, upper respiratory tract infection, otitis media, and were mostly mild to moderate in severity. Serious infections were observed in 9% of patients receiving adalimumab in the study and included dental caries, rotavirus gastroenteritis, and varicella.
In Study JIA-II, non-serious allergic reactions were observed in 6% of patients and included intermittent urticaria and rash, which were all mild in severity.
Psoriatic Arthritis and Ankylosing Spondylitis Clinical Studies
Adalimumab has been studied in 395 patients with psoriatic arthritis (PsA) in two placebo- controlled trials and in an open label study and in 393 patients with ankylosing spondylitis (AS) in two placebo-controlled studies [
Crohn’s Disease Clinical Studies
Adults: The safety profile of adalimumab in 1478 adult patients with Crohn’s disease from four placebo-controlled and two open-label extension studies [see Clinical Studies (was similar to the safety profile seen in patients with RA.
Pediatric Patients 6 Years to 17 Years: The safety profile of adalimumab in 192 pediatric patients from one double-blind study (Study PCD-I) and one open-label extension study [see Clinical Studies (was similar to the safety profile seen in adult patients with Crohn’s disease.
During the 4-week open label induction phase of Study PCD-I, the most common adverse reactions occurring in the pediatric population treated with adalimumab were injection site pain and injection site reaction (6% and 5%, respectively).
A total of 67% of children experienced an infection while receiving adalimumab in Study PCD-I. These included upper respiratory tract infection and nasopharyngitis.
A total of 5% of children experienced a serious infection while receiving adalimumab in Study PCD-I. These included viral infection, device related sepsis (catheter), gastroenteritis, H1N1 influenza, and disseminated histoplasmosis.
In Study PCD-I, allergic reactions were observed in 5% of children which were all non-serious and were primarily localized reactions.
Ulcerative Colitis Clinical Studies
Adults: The safety profile of adalimumab in 1010 adult patients with ulcerative colitis (UC) from two placebo-controlled studies and one open-label extension study [see Clinical Studies ( was similar to the safety profile seen in patients with RA.
Plaque Psoriasis Clinical Studies
Adalimumab has been studied in 1696 subjects with plaque psoriasis (Ps) in placebo-controlled and open-label extension studies
Hidradenitis Suppurativa Clinical Studies
Adalimumab has been studied in 727 subjects with hidradenitis suppurativa (HS) in three placebo- controlled studies and one open-label extension study
Flare of HS, defined as ≥25% increase from baseline in abscesses and inflammatory nodule counts and with a minimum of 2 additional lesions, was documented in 22 (22%) of the 100 subjects who were withdrawn from adalimumab treatment following the primary efficacy timepoint in two studies.
Uveitis Clinical Studies
Adalimumab has been studied in 464 adult patients with uveitis (UV) in placebo-controlled and open-label extension studies (Study PUV-I) [
3.2Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of adalimumab or of other adalimumab products.
There are two assays that have been used to measure anti-adalimumab antibodies. With the ELISA, antibodies to adalimumab could be detected only when serum adalimumab concentrations were < 2 mcg/mL. The ECL assay can detect anti-adalimumab antibody titers independent of adalimumab concentrations in the serum samples. The incidence of anti‑adalimumab antibody (AAA) development in patients treated with adalimumab are presented in
n: number of patients with anti-adalimumab antibody; NR: not reported; NA: Not applicable (not performed)
Rheumatoid Arthritis and Psoriatic Arthritis: Patients in Studies RA-I, RA-II, and RA-III were tested at multiple time points for antibodies to adalimumab using the ELISA during the 6- to 12-month period. No apparent correlation of antibody development to adverse reactions was observed. With monotherapy, patients receiving every other week dosing may develop antibodies more frequently than those receiving weekly dosing. In patients receiving the recommended dosage of 40 mg every other week as monotherapy, the ACR 20 response was lower among antibody-positive patients than among antibody-negative patients. The long-term immunogenicity of adalimumab products is unknown.
3.3Postmarketing Experience
The following adverse reactions have been identified during post-approval use of adalimumab products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to adalimumab products exposure.
Gastrointestinal disorders: Diverticulitis, large bowel perforations including perforations associated with diverticulitis and appendiceal perforations associated with appendicitis, pancreatitis
General disorders and administration site conditions: Pyrexia
Hepato-biliary disorders: Liver failure, hepatitis
Immune system disorders: Sarcoidosis
Neoplasms benign, malignant and unspecified (including cysts and polyps): Merkel Cell Carcinoma (neuroendocrine carcinoma of the skin)
Nervous system disorders: Demyelinating disorders (e.g., optic neuritis, Guillain-Barré syndrome), cerebrovascular accident
Respiratory disorders: Interstitial lung disease, including pulmonary fibrosis, pulmonary embolism
Skin reactions: Stevens Johnson Syndrome, cutaneous vasculitis, erythema multiforme, new or worsening psoriasis (all sub-types including pustular and palmoplantar), alopecia, lichenoid skin reaction
Vascular disorders: Systemic vasculitis, deep vein thrombosis
4OVERDOSAGE
Doses up to 10 mg/kg have been administered to patients in clinical trials without evidence of dose-limiting toxicities. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment instituted immediately.
5DESCRIPTION
Adalimumab-aacf is a tumor necrosis factor blocker. Adalimumab-aacf is a recombinant human IgG1 monoclonal antibody created using phage display technology resulting in an antibody with human derived heavy and light chain variable regions and human IgG1:k constant regions. Adalimumab-aacf is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary (CHO)) expression system and is purified by a process that includes specific viral inactivation and removal steps. It consists of 1330 amino acids and has a molecular weight of approximately 148 kilodaltons.
IDACIO (adalimumab-aacf) injection is supplied as a sterile, preservative-free solution for subcutaneous administration. The drug product is supplied as either a single-dose, prefilled pen (IDACIO Pen), as a single-dose, 1 mL or prefilled glass syringe or as a single dose institutional use vial kit. Enclosed within the pen is a single-dose, 1 mL prefilled glass syringe. The solution of IDACIO is clear and colorless to pale yellow, with a pH of about 5.2.
Each 40 mg/0.8 mL prefilled syringe or prefilled pen, or institutional use vial kit delivers 0.8 mL (40 mg) of drug product. Each 0.8 mL of IDACIO contains adalimumab-aacf (40 mg) and glacial acetic acid (0.5 mg), trehalose (54.8 mg), polysorbate 80 (0.8 mg), sodium chloride (2.3 mg), and Water for Injection. Sodium hydroxide is added to adjust pH.
6REFERENCES
1. National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 17 Registries, 2000-2007.
7HOW SUPPLIED/STORAGE AND HANDLING
IDACIO (adalimumab-aacf) injection is supplied as a preservative-free, sterile, clear and colorless to pale yellow solution for subcutaneous administration. The following packaging configurations are available.
- IDACIO Pen Carton - 40 mg/0.8 mL (2 count)
IDACIO is supplied in a carton containing 2 alcohol preps and one tray. The tray contains two single-dose pens, each containing a 1 mL prefilled glass syringe with a 29 gauge staked ½ inch needle, providing 40 mg/0.8 mL of IDACIO. The syringe plunger stopper and needle cover are not made with natural rubber latex. The NDC number is 65219-554-08
- IDACIO Pen 40 mg/0.8 mL - Starter Package for Plaque Psoriasis or Uveitis (4 Count)
IDACIO is supplied in a carton containing 4 alcohol preps and 2 trays (Starter Package for Plaque Psoriasis or Uveitis). Each tray contains two single-dose pens, each pen containing a 1 mL prefilled glass syringe with a 29 gauge staked ½ inch needle, providing 40 mg/0.8 mL of IDACIO. The syringe plunger stopper and needle cover are not made with natural rubber latex. The NDC number is 65219-554-28.
- IDACIO Pen 40 mg/0.8 mL - Starter Package for Crohn's Disease, Ulcerative Colitis, or Hidradenitis Suppurativa (6 Count)
IDACIO is supplied in a carton containing 6 alcohol preps and 3 trays (Starter Package for Crohn’s Disease, Ulcerative Colitis, or Hidradenitis Suppurativa). Each tray contains two single-dose pens, each pen containing a 1 mL prefilled glass syringe with a 29 gauge staked ½ inch 65219-628-89needle, providing 40 mg/0.8 mL of IDACIO. The syringe plunger stopper and needle cover are not made with natural rubber latex. The NDC number is 65219-554-38.
- IDACIO Prefilled Syringe Carton - 40 mg/0.8 mL (2 count)
IDACIO is supplied in a carton containing 2 alcohol preps and one tray. The tray contains two single-dose, 1 mL prefilled glass syringes with a 29 gauge staked ½ inch needle, each syringe providing 40 mg/0.8 mL of IDACIO. The syringe plunger stopper and needle cover are not made with natural rubber latex. The NDC number is 65219-556-18.
- IDACIO Single-Dose Institutional Use Vial Kit - 40 mg/0.8 mL.
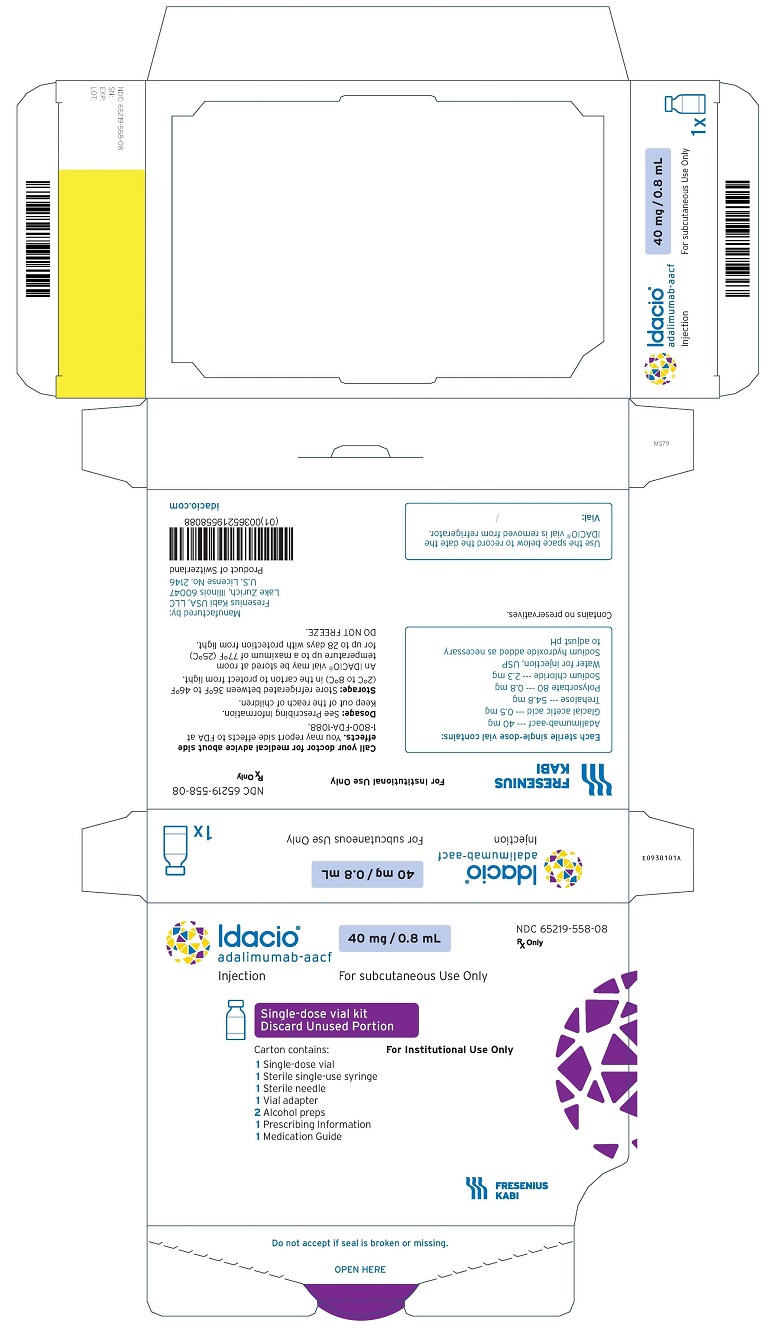
IDACIO is supplied in a carton containing 1 sterile single-use syringe, 1 sterile needle, 1 vial adapter, 2 alcohol preps and 1 glass vial providing 40 mg/0.8 mL of IDACIO. The vial stopper is not made with natural rubber latex. The NDC number is 65219-558-08.
Storage and Stability
Do not use beyond the expiration date on the container. IDACIO must be refrigerated at 36°F to 46°F (2°C to 8°C). DO NOT FREEZE. Do not use if frozen even if it has been thawed.
Store in original carton until time of administration to protect from light.
If needed, for example when traveling, IDACIO may be stored at room temperature up to a maximum of 77°F (25°C) for a period of up to 28 days, with protection from light. IDACIO should be discarded if not used within the 28-day period. Record the date when IDACIO is first removed from the refrigerator in the spaces provided on the carton.
Do not store IDACIO in extreme heat or cold.
8PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Infections
Inform patients that IDACIO may lower the ability of their immune system to fight infections. Instruct patients of the importance of contacting their doctor if they develop any symptoms of infection, including tuberculosis, invasive fungal infections, and reactivation of hepatitis B virus infections
Malignancies
Counsel patients about the risk of malignancies while receiving IDACIO
Hypersensitivity Reactions
Advise patients to seek immediate medical attention if they experience any symptoms of severe hypersensitivity reactions.
Other Medical Conditions
Advise patients to report any signs of new or worsening medical conditions such as congestive heart failure, neurological disease, autoimmune disorders, or cytopenias. Advise patients to report any symptoms suggestive of a cytopenia such as bruising, bleeding, or persistent fever
Instructions on Injection Technique
Inform patients that the first injection is to be performed under the supervision of a qualified health care professional. If a patient or caregiver is to administer IDACIO, instruct them in injection techniques and assess their ability to inject subcutaneously to ensure the proper administration of IDACIO
For patients who will use the IDACIO Pen, tell them to:
- Remove the needle cap of the Pen and push and hold the Pen firmly against the skin on the chosen injection site.
- Activate Pen by pressing the injection button. The click means the start of the injection.
- Keep holding the IDACIO Pen against their skin until the injection has finished.
- Will know that the injection has finished when the syringe plunger moves all the way down to the bottom of the transparent syringe housing to enable the safety guard to cover the needle
Instruct patients to dispose of their used needles and syringes or used Pen in a FDA-cleared sharps disposal container immediately after use.
Instruct patients that when their sharps disposal container is almost full, they will need to follow their community guidelines for the correct way to dispose of their sharps disposal container. Instruct patients that there may be state or local laws regarding disposal of used needles and syringes. Refer patients to the FDA’s website at http://www.fda.gov/safesharpsdisposal for more information about safe sharps disposal, and for specific information about sharps disposal in the state that they live in.
Instruct patients not to dispose of their used sharps disposal container in their household trash unless their community guidelines permit this. Instruct patients not to recycle their used sharps disposal container.
Manufactured by: Fresenius Kabi USA, LLC
Lake Zurich, IL 60047, U.S.A
US License Number 2146
9Principal Display Panel –40 mg/ 0.8 mL Single-Dose Institutional Use-Carton-Label
Idacio
adalimumab-aacf
Injection
40mg/ 0.8mL
NDC 65219-558-08
Rx Only
For subcutaneous Use Only
Single-dose vial kit
Discard Unused Portion
Carton contains: For Institutional Use Only
1 Single-dose vial
1 Sterile single-use syringe
1. Sterile needle
1 Vial adapter
2 Alcohol preps
1 Prescribing information
1 Medication guide
FRESENIUS KABI
10Principal Display Panel –40 mg/ 0.8 mL Single-Dose Institutional Use-Vial-Label
Idacio 40mg/ 0.8mL NDC 65219-562-01
adalimumab-aacf Injection
For subcutaneous Use Only
EXP:
Lot:
US License No. 2146
Fresenius Kabi USA, LLC
