Aromasin
What is Aromasin (Exemestane)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The primary goal of this study is to evaluate the effectiveness of elacestrant versus standard endocrine therapy in participants with node-positive, Estrogen Receptor-positive (ER+), Human Epidermal Growth Factor-2 negative (HER2-) early breast cancer with high risk of recurrence.
Summary: The purpose of this open-label, multicenter, phase IIIb, single-arm study is to characterize the efficacy and safety of the combination of ribociclib and standard adjuvant endocrine therapy (ET) on invasive breast cancer-free survival (iBCFS), in a close to clinical practice patient population with HR-positive (HR+), HER2-negative (HER2-), Anatomic Stage Group III, IIB, and a subset of Stage IIA E...
Summary: The primary objective of the study is to measure efficacy of saruparib (AZD5305) plus camizestrant compared with physician's choice CDK4/6i plus ET in patients with BRCA1, BRCA2, or PALB2m, HR-positive, HER2-negative (defined as IHC 0, 1+, 2+/ ISH non-amplified) advanced breast cancer
Related Latest Advances
Brand Information
- Reductions in Bone Mineral Density (BMD)
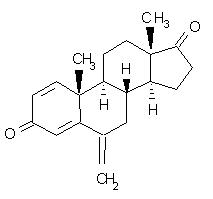
exemestane tablets

