-111 Pentetreotide
What is Octreoscan (-111 Pentetreotide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Between 10% and 15% of patients with endogenous hypercortisolism (Cushing syndrome) have ectopic (non-pituitary) production of adrenocorticotropin hormone (ACTH) that causes cortisol excess. In approximately 50% of these patients, the tumoral source of ACTH cannot be found initially despite very detailed and extensive imaging, including studies such as computed tomography, magnetic resonance imagi...
Summary: A \[68\]Ga-HA-DOTATATE PET/CT or PET/MRI scan is a nuclear medicine test used to create pictures of the whole body that will show where somatostatin receptors are found, including on tumours. Somatostatin receptors are found on most neuroendocrine tumours (NETs), and some other types of tumours. Currently at the Cross Cancer Institute, most patients with suspected somatostatin positive tumours (e....
Summary: This clinical trial is a pragmatic study aiming to evaluate the innocuity/safety profile of the PET radiotracer 68Ga-DOTA-TATE, and to establish the procedure as a routine standard-of-care diagnostic tool for all neuro-endocrine cancer patients. It is a single-center study, but with recruitment across all Canada. The trial is prospective, non-randomized, open-label and with no control group. The s...
Related Latest Advances
Brand Information
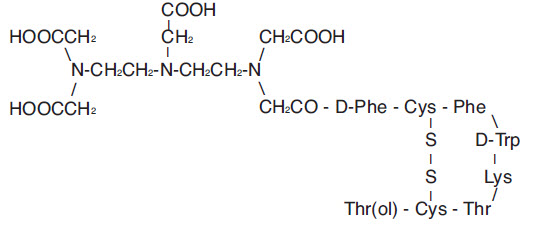
- A 10-mL Octreoscan Reaction Vial which contains a lyophilized mixture of:
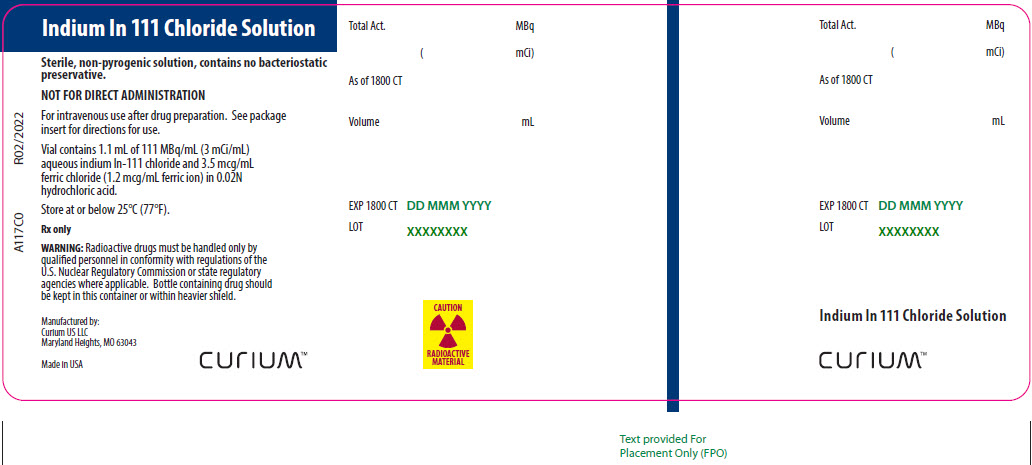
- A 10-mL vial of Indium In 111 Chloride Solution, which contains 1.1 mL or 111 MBq/mL (3 mCi/mL) Indium In 111 chloride in 0.02 N HCl at time of calibration. The vial also contains ferric chloride at a concentration of 3.5 mcg/mL (ferric ion, 1.2 mcg/mL). The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
- All transfers and penetrations of the vial stoppers by a needle must use aseptic technique.
- Wear waterproof gloves during the entire procedure and while withdrawing the patient-dose from the Octreoscan Reaction Vial.
- Transfer Indium In 111 Chloride Solution with an adequately shielded, sterile syringe using the transfer needle in the kit.
- Adequate shielding should be maintained at all times until the preparation is administered to the patient, disposed of in an approved manner, or allowed to decay to safe levels of radioactivity. A shielded, sterile syringe should be used for withdrawing and injecting the preparation.
- Do not inject into TPN administration bags or their intravenous lines.
- Place the Octreoscan Reaction Vial in a lead dispensing shield (of minimum wall thickness 1/4 inch) fitted with a lid.
- Swab the rubber stopper of the reaction vial with an appropriate antiseptic and allow the vial to dry.
- Aseptically remove the contents of the Indium In 111 Chloride Solution vial using the needle provided and a shielded, sterile syringe.
- Inject the Indium In 111 Chloride Solution into the Octreoscan Reaction Vial.
- Gently swirl the Octreoscan Reaction Vial until the lyophilized pellet is completely dissolved.
- Incubate the Indium In 111 Pentetreotide Injection at or below 25°C (77°F) for a minimum of 30 minutes. Note: A 30 minute incubation time is required. Shorter incubation periods may result in inadequate radiolabeling.
- Using proper shielding, visually inspect the vial contents. The solution should be clear, colorless, and free of particulate matter. If not, the solution should not be used. It should be disposed in a safe and approved manner.
- Assay the Indium In 111 Pentetreotide Injection using a suitably calibrated ionization chamber. Record the date, time, total activity, and patient identifier (e.g., patient name and number) on the radioassay information label and affix the label to the lead dispensing shield.
- The radiolabeling yield of the reconstituted solution should be checked before administration to the patient, according to the instructions given below. If the radiochemical purity is less than 90%, the product should not be used.
- Store the reaction vial containing the Indium In 111 Pentetreotide Injection at controlled room temperature 20° to 25°C (68° to 77°F) until use. The Indium In 111 Pentetreotide Injection must be used within 6 hours of preparation.
- If desired, the preparation can be diluted to a maximum volume of 3 mL with 0.9% Sodium Chloride Injection, USP immediately prior to injection. The sample should be drawn up into a shielded, sterile syringe and administered to the patient.
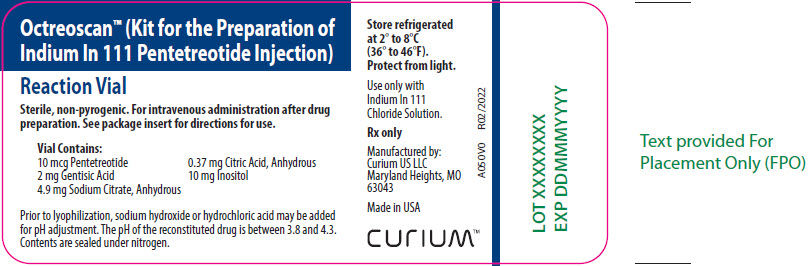
Vial Contains:
10 mcg Pentetreotide
2 mg Gentisic Acid
4.9 mg Sodium Citrate, Anhydrous
0.37 mg Citric Acid, Anhydrous
10 mg Inositol
Prior to lyophilization, sodium hydroxide or hydrochloric acid may be added for pH adjustment. The pH of the reconstituted drug is between 3.8 and 4.3. Contents are sealed under nitrogen.
For intravenous use after drug preparation. See package insert for directions for use.
Vial contains 1.1 mL of 111 MBq/mL (3 mCi/mL) aqueous indium In-111 chloride and 3.5 mcg/mL ferric chloride (1.2 mcg/mL ferric ion) in 0.02N hydrochloric acid.
Store at or below 25°C (77°F).
Rx only
WARNING: Radioactive drugs must be handled only by qualified personnel in conformity with regulations of the U.S. Nuclear Regulatory Commission or state regulatory agencies where applicable. Bottle containing drug should be kept in this container or within heavier shield.
Manufactured by:
Curium US LLC
Maryland Heights, MO 63043