Brand Name
Uloric
Generic Name
Febuxostat
View Brand Information FDA approval date: February 13, 2009
Classification: Xanthine Oxidase Inhibitor
Form: Tablet, Film
What is Uloric (Febuxostat)?
Febuxostat tablets are xanthine oxidase inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable. Limitations of Use : Febuxostat tablets are not recommended for the treatment of asymptomatic hyperuricemia. Febuxostat tablets are xanthine oxidase inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable. Limitations of Use: Febuxostat tablets are not recommended for the treatment of asymptomatic hyperuricemia.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ULORIC (febuxostat)
WARNING: CARDIOVASCULAR DEATH
Gout patients with established cardiovascular (CV) disease treated with ULORIC had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study
Consider the risks and benefits of ULORIC when deciding to prescribe or continue patients on ULORIC. ULORIC should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable
1INDICATIONS AND USAGE
ULORIC is a xanthine oxidase (XO) inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable.
2DOSAGE FORMS AND STRENGTHS
- 40 mg tablets, light green to green, round, debossed with "TAP" and "40"
- 80 mg tablets, light green to green, teardrop shaped, debossed with "TAP" and "80"
3CONTRAINDICATIONS
ULORIC is contraindicated in patients being treated with azathioprine or mercaptopurine
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the prescribing information:
- Cardiovascular Death
- Hepatic Effects
- Serious Skin Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Phase 2 and 3 clinical studies, a total of 2757 patients with hyperuricemia and gout were treated with ULORIC 40 mg or 80 mg daily. For ULORIC 40 mg, 559 patients were treated for ≥6 months. For ULORIC 80 mg, 1377 patients were treated for ≥6 months, 674 patients were treated for ≥1 year and 515 patients were treated for ≥2 years. In the CARES study, a total of 3098 patients were treated with ULORIC 40 mg or 80 mg daily; of these, 2155 patients were treated for ≥1 year and 1539 were treated for ≥2 years
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of ULORIC. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: agranulocytosis, eosinophilia.
Hepatobiliary Disorders: hepatic failure (some fatal), jaundice, serious cases of abnormal liver function test results, liver disorder.
Immune System Disorders: anaphylaxis, anaphylactic reaction.
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis.
Psychiatric Disorders: psychotic behavior including aggressive thoughts.
Renal and Urinary Disorders: tubulointerstitial nephritis.
Skin and Subcutaneous Tissue Disorders: generalized rash, Stevens-Johnson Syndrome, hypersensitivity skin reactions, erythema multiforme, drug reaction with eosinophilia and systemic symptoms, toxic epidermal necrolysis.
5OVERDOSAGE
ULORIC was studied in healthy patients in doses up to 300 mg daily for seven days without evidence of dose-limiting toxicities. No overdose of ULORIC was reported in clinical studies. Patients should be managed by symptomatic and supportive care should there be an overdose.
6DESCRIPTION
ULORIC (febuxostat) is a xanthine oxidase inhibitor. The active ingredient in ULORIC is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of 316.38. The empirical formula is C
The chemical structure is:
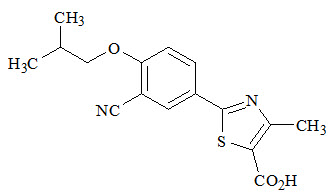
Febuxostat is a non-hygroscopic, white crystalline powder that is freely soluble in dimethylformamide; soluble in dimethylsulfoxide; sparingly soluble in ethanol; slightly soluble in methanol and acetonitrile; and practically insoluble in water. The melting range is 205°C to 208°C.
ULORIC tablets for oral use contain the active ingredient, febuxostat, and are available in two dosage strengths, 40 mg and 80 mg. Inactive ingredients include hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium croscarmellose. ULORIC tablets are coated with Opadry II, green.
7CLINICAL STUDIES
A serum uric acid level of less than 6 mg/dL is the goal of antihyperuricemic therapy and has been established as appropriate for the treatment of gout.
7.1Management of Hyperuricemia in Gout
The efficacy of ULORIC was demonstrated in three randomized, double-blind, controlled trials in patients with hyperuricemia and gout. Hyperuricemia was defined as a baseline serum uric acid level ≥8 mg/dL.
Study 1 (NCT00430248) randomized patients to: ULORIC 40 mg daily, ULORIC 80 mg daily, or allopurinol (300 mg daily for patients with estimated creatinine clearance (Cl
Study 2 (NCT00174915) randomized patients to: placebo, ULORIC 80 mg daily, ULORIC 120 mg daily, ULORIC 240 mg daily or allopurinol (300 mg daily for patients with a baseline serum creatinine ≤1.5 mg/dL or 100 mg daily for patients with a baseline serum creatinine greater than 1.5 mg/dL and ≤2 mg/dL). The duration of Study 2 was six months.
Study 3 (NCT00102440), a year study, randomized patients to: ULORIC 80 mg daily, ULORIC 120 mg daily, or allopurinol 300 mg daily. Patients who completed Study 2 and Study 3 were eligible to enroll in a Phase 3 long-term extension study in which patients received treatment with ULORIC for over three years.
In all three studies, patients received naproxen 250 mg twice daily or colchicine 0.6 mg once or twice daily for gout flare prophylaxis. In Study 1 the duration of prophylaxis was six months; in Study 2 and Study 3 the duration of prophylaxis was eight weeks.
The efficacy of ULORIC was also evaluated in a 4-week dose ranging study which randomized patients to: placebo, ULORIC 40 mg daily, ULORIC 80 mg daily, or ULORIC 120 mg daily. Patients who completed this study were eligible to enroll in a long-term extension study in which patients received treatment with ULORIC for up to five years.
Patients in these studies were representative of the patient population for which ULORIC use is intended. Table 2 summarizes the demographics and baseline characteristics for the patients enrolled in the studies.
7.2Cardiovascular Safety Study
A randomized, double-blind, allopurinol-controlled CV outcomes study (CARES) was conducted to evaluate the CV risk of ULORIC (NCT01101035). The study compared the risk of MACE between patients treated with ULORIC (N=3098) and allopurinol-treated patients (N=3092). The primary endpoint was the time to first occurrence of a MACE defined as the composite of CV death, nonfatal MI, nonfatal stroke, or unstable angina with urgent coronary revascularization. The study was designed to exclude a prespecified risk margin of 1.3 for the hazard ratio of MACE. An independent committee conducted a blinded evaluation of serious CV adverse events according to predefined criteria (adjudication) for determination of MACE. The study was event driven and patients were followed until a sufficient number of primary outcome events accrued. The median on-study follow-up time was 2.6 years.
Patients randomized to ULORIC initially received 40 mg once daily which was increased to 80 mg once daily, if their sUA was ≥6mg/dL at Week 2. For patients randomized to allopurinol, those who had normal renal function or mild renal impairment (estimated creatinine clearance (eCl
The mean age of the population was 65 years (range: 44 to 93 years). Most patients were male (84%) and Caucasian (69%). Patients had a diagnosis of gout for approximately 12 years, a mean baseline sUA of 8.7 mg/dL, and 90% had experienced at least one gout flare in the past year. CV history included MI (39%), hospitalization for unstable angina (28%), cardiac revascularization (37%), and stroke (14%). The most prevalent comorbid conditions were hypertension (92%), hyperlipidemia (87%), diabetes mellitus (55%), diabetes mellitus with micro- or macrovascular disease (39%), and renal impairment [92% with an eCl
Table 5 shows the study results for the primary MACE composite endpoint and its individual components. For the composite primary endpoint, the ULORIC group was non-inferior compared with the allopurinol group. The rates of nonfatal MI, stroke, and unstable angina with urgent coronary revascularization were similar. There was a higher rate of CV deaths in patients treated with ULORIC (134 CV deaths; 1.5 per 100 PY) than in allopurinol-treated patients (100 CV deaths; 1.1 per 100 PY). Sudden cardiac death was the most common cause of adjudicated CV deaths in the ULORIC group (83 of 3,098; 2.7%) as compared to the allopurinol group (56 of 3,092; 1.8%). The biological plausibility of CV death associated with ULORIC is unclear.
All-cause mortality was higher in the ULORIC group (243 deaths [7.8%]; 2.6 per 100 PY) than the allopurinol group (199 deaths [6.4%]; 2.2 per 100 PY) [Hazard Ratio: 1.22, 95% CI: 1.01, 1.47], due to a higher rate of CV deaths.
8HOW SUPPLIED/STORAGE AND HANDLING
ULORIC 40 mg tablets are light green to green in color, round, debossed with "TAP" on one side and "40" on the other side and supplied as:
ULORIC 80 mg tablets are light green to green in color, teardrop shaped, debossed with "TAP" on one side and "80" on the other side and supplied as:
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
10PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label
NDC 64764-918-30
Uloric
40 mg
Dispense the accompanying
11PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle Label
NDC 64764-677-30
Uloric
80 mg
Dispense the accompanying
