Generic Name
Baclofen
Brand Names
Lyvispah, Ozobax, Fleqsuvy, Lioresal, Gablofen
FDA approval date: July 21, 1988
Classification: gamma-Aminobutyric Acid-ergic Agonist
Form: Injection, Tablet, Kit, Granule, Suspension, Solution
What is Lyvispah (Baclofen)?
Baclofen tablets USP are useful for the alleviation of signs and symptoms of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity. Patients should have reversible spasticity so that baclofen tablet treatment will aid in restoring residual function. Baclofen tablets USP may also be of some value in patients with spinal cord injuries and other spinal cord diseases. Baclofen tablets USP are not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders. The efficacy of baclofen tablets in stroke, cerebral palsy, and Parkinson’s disease has not been established and, therefore, it is not recommended for these conditions.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
LYVISPAH (baclofen)
1INDICATIONS AND USAGE
LYVISPAH is indicated for the treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity.
LYVISPAH may also be of some value in patients with spinal cord injuries and other spinal cord diseases.
Limitations of Use
LYVISPAH is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders.
2DOSAGE FORMS AND STRENGTHS
Oral Granules: 5 mg, 10 mg, or 20 mg baclofen as white to off-white, strawberry flavored granules in a single dose packet.
3CONTRAINDICATIONS
LYVISPAH is contraindicated in patients with hypersensitivity to baclofen.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Adverse Reactions from Abrupt Withdrawal of LYVISPAH
- Neonatal Withdrawal Symptoms
- Drowsiness and Sedation
- Poor Tolerability in Stroke Patients
- Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States
- Exacerbation of Autonomic Dysreflexia
- Exacerbation of Epilepsy
- Posture and Balance Effects
- Ovarian Cysts
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reaction is transient drowsiness. In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen compared to 36% of those in the placebo group. Other common adverse reactions (up to 15%) are dizziness and weakness.
Adverse reactions with a frequency of ≥1% are listed in Table 1.
Table 1. Common (≥1%) Adverse Reactions in Patients Treated with Baclofen for Spasticity
The following adverse reactions not included in Table 1, classified by body system, were also reported:
Neuropsychiatric: euphoria, excitement, depression, hallucinations, paresthesia, muscle pain, tinnitus, slurred speech, coordination disorder, tremor, rigidity, dystonia, ataxia, blurred vision, nystagmus, strabismus, miosis, mydriasis, diplopia, dysarthria, epileptic seizure Cardiovascular: dyspnea, palpitation, chest pain, syncope
Gastrointestinal: dry mouth, anorexia, taste disorder, abdominal pain, vomiting, diarrhea, and positive test for occult blood in stool
Genitourinary: enuresis, urinary retention, dysuria, impotence, inability to ejaculate, nocturia, hematuria
Other: rash, pruritus, ankle edema, excessive perspiration, weight gain, nasal congestion
The following laboratory tests have been found to be abnormal in patients receiving baclofen: increased SGOT, elevated alkaline phosphatase, and elevation of blood sugar.
5DESCRIPTION
LYVISPAH (baclofen) oral granules is a gamma-aminobutyric acid (GABA-ergic) agonist available as 5 mg, 10 mg, or 20 mg of baclofen oral granules in a packet. Its chemical name is 4-amino-3-(4- chlorophenyl)-butanoic acid and its structural formula is:
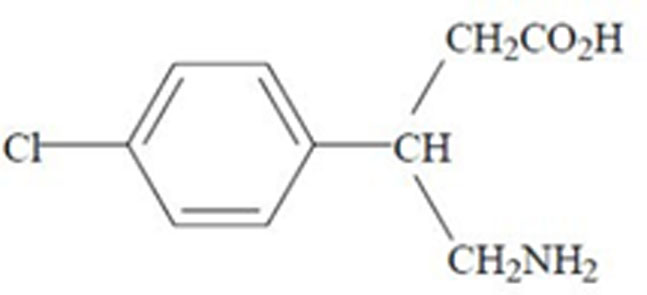
Molecular formula is C10H12ClNO2 Molecular Weight is 213.66.
Baclofen USP is a white to off-white, odorless or practically odorless crystalline powder. It is slightly soluble in water, very slightly soluble in methanol, and insoluble in chloroform.
LYVISPAH (baclofen) oral granules inactive ingredients include amino methacrylate copolymer, calcium stearate, colloidal silicon dioxide, crospovidone, hypromellose, mannitol, saccharin sodium, strawberry flavor, talc, and xylitol.
6CLINICAL STUDIES
The efficacy of LYVISPAH is based upon a bioavailability study in healthy adults comparing baclofen oral tablets to LYVISPAH
7PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (
General Administration Instructions
Instruct patient or caregiver to carefully open the LYVISPAH packet and empty the entire contents, to obtain the prescribed amount of medication. Packet contents can be administered directly into the mouth. The contents of the packet can be swallowed or will dissolve in the mouth. LYVISPAH may be taken with liquids or can also be administered in soft foods if needed
Administration Instructions with Soft Foods
LYVISPAH can also be administered by mouth as a mixture with liquids or soft foods, such as apple sauce, yogurt, or pudding. One packet can be mixed with up to 15 mL (one tablespoonful) of soft food. The mixture should be administered no more than 2 hours after mixing
Administration Instructions via Feeding Tubes
LYVISPAH can be administered via enteral feeding tubes, such a nasogastric (NG), gastrostomy (G), percutaneous endoscopic gastrostomy (PEG), and gastrojejunostomy (GJ) tubes.
- Flush the feeding tube with up to 15 mL (one tablespoonful) of water.
- Open and empty the full contents of one packet of LYVISPAH in 15 mL (one tablespoonful) of the preferred liquid. Stir the suspension to ensure all granules are wetted.
- Draw up the suspension of granules into a dosing syringe immediately after stirring and administer the dose via the feeding tube. Administer no longer than two hours after mixing.
- Refill the dosing syringe with 15 mL (one tablespoonful) of water and flush the feeding tube with the remaining contents.
- Any unused suspensions should be discarded.
Risks Related to Sudden Withdrawal of LYVISPAH
Advise patients and caregivers not to discontinue use of LYVISPAH without consulting with their healthcare provider because sudden withdrawal of LYVISPAH can result in serious complications that include hallucinations, seizures, high fever, confusion, muscle stiffness, multiple organ-system failure, and death
Neonatal Withdrawal Symptoms
Advise patients to notify their healthcare provider if they are pregnant, plan to become pregnant, or plan to breastfeed
Increased Risk of Drowsiness with Alcohol and Other CNS Depressants
Advise patients that LYVISPAH may cause drowsiness, and that they should avoid the operation of automobiles or other dangerous machinery, or activities made hazardous by decreased alertness when starting LYVISPAH or increasing the dose of LYVISPAH until they know how the drug affects them
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 04-2023-01
8PATIENT INFORMATION
This Patient Information has been approved by the U.S. Food and Drug Administration
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 04-2023-01
9INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
LYVISPAH (lye vis' pah) (baclofen)
oral granules
Important information to know before taking LYVISPAH
LYVISPAH can be taken without liquids.
If needed, LYVISPAH can be mixed with liquids or soft foods. LYVISPAH should be taken within 2 hours of mixing into liquids or soft foods.
How to take LYVISPAH? Opening LYVISPAH
Shake the packet to distribute settled granules to the bottom of the packet. Carefully open the LYVISPAH packet by cutting the dotted lines across the top of the packet (see the figure).

Taking LYVISPAH by emptying the granules into the mouth
Empty all the granules in the LYVISPAH packet directly into your mouth. The granules will dissolve in your mouth or can be swallowed. After taking LYVISPAH, you can drink water if needed to swallow any granules left in your mouth.
Taking LYVISPAH with liquids or soft foods
LYVISPAH can be taken or given by mouth as a mixture with liquids such as milk or apple juice. LYVISPAH can also be taken or given by mouth as a mixture with soft foods such as apple sauce, yogurt, or pudding.
- Open 1 packet of LYVISPAH (see the figure above).
- Empty the full contents of the LYVISPAH packet into 1 tablespoon (15 mL) liquid or soft food and mix it.
- Take LYVISPAH within 2 hours of mixing into liquids or soft foods.
- If more than 1 packet of LYVISPAH is needed for your prescribed dose, mix each packet with a separate amount of liquid or soft food.
Giving LYVISPAH through a feeding tube
LYVISPAH can be given through enteral feeding tubes such as nasogastric (NG), gastrostomy (G), percutaneous endoscopic gastrostomy (PEG), and gastrojejunostomy (GJ) tubes.
- Flush the feeding tube with up to 1 tablespoon (15 mL) of water using a catheter tip syringe.
- Open and empty the full contents of 1 packet of LYVISPAH into a clean container and mix with 1 tablespoon (15 mL) of liquid (apple juice or milk).
- Stir the mixture to make sure all the granules are wet.
- Draw up the mixture of granules into a dosing syringe right away after stirring.
- Give the dose of LYVISPAH through the feeding tube within 2 hours after mixing. If the mixture is in the dosing syringe for 15 minutes and not given, turn the dosing syringe upside down 3 times before you give the dose.
- Fill the dosing syringe with 1 tablespoon (15 mL) of water and flush the feeding tube.
- If more than 1 packet of LYVISPAH is needed for the prescribed dose, mix each packet with a separate amount of liquid.
- Throw away (dispose of) any unused mixture.
Important Information
Take the entire packet of LYVISPAH to get the prescribed dose.
Keep LYVISPAH and all medicines out of the reach of children.
This Patient Information and Instructions for Use have been approved by the US Food and Drug Administration.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 04-2023-01
10PRINCIPAL DISPLAY PANEL
