Brand Name
Welchol
Generic Name
Colesevelam
View Brand Information FDA approval date: January 01, 2010
Classification: Bile Acid Sequestrant
Form: Tablet, Powder, For
What is Welchol (Colesevelam)?
Colesevelam hydrochloride tablets is a bile acid sequestrant indicated as an adjunct to diet and exercise to · reduce elevated low-density lipoprotein cholesterol in adults with primary hyperlipidemia.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Metabolic Effects of Endogenous Bile Acids After Gastric Bypass Surgery
Summary: Non-randomized, open-label, parallel-group clinical study evaluating the effects of endogenous bile acids on changes in plasma fibroblast growth factor-19 (FGF-19) and glucose metabolism by extended depletion of circulating bile acids using colesevelam as an experimental tool in subjects operated with gastric by-pass (RYGB).
Related Latest Advances
Brand Information
Welchol (colesevelam hydrochloride)
1DOSAGE FORMS AND STRENGTHS
- Tablets: 625 mg tablets are off-white, oval, film-coated and imprinted with "Sankyo" and "C01" on one side.
- For Oral Suspension: 3.75 gram packet containing a white to pale yellow powder with yellow granules.
2CONTRAINDICATIONS
WELCHOL is contraindicated in patients with:
- Serum TG concentrations >500 mg/dL
- History of hypertriglyceridemia-induced pancreatitis
- A history of bowel obstruction
3ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Hypertriglyceridemia and Pancreatitis
- Gastrointestinal Obstruction
- Vitamin K or Fat-Soluble Vitamin Deficiencies
3.1Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in clinical studies of another drug and may not reflect the rates observed in practice.
3.2Post-marketing Experience
The following additional adverse reactions have been identified during post-approval use of WELCHOL. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse Reactions Resulting from Drug Interactions [see Increased seizure activity or decreased phenytoin levels in patients receiving phenytoin, reduced International Normalized Ratio (INR) in patients receiving warfarin therapy, and elevated thyroid-stimulating hormone (TSH) in patients receiving thyroid hormone replacement therapy
Gastrointestinal: Bowel obstruction (in patients with a history of bowel obstruction or resection), dysphagia or esophageal obstruction (occasionally requiring medical intervention), fecal impaction, pancreatitis, abdominal distension, exacerbation of hemorrhoids, and increased transaminases
Laboratory Abnormalities: Hypertriglyceridemia
4OVERDOSAGE
WELCHOL is not absorbed and the risk of systemic toxicity is low. Excessive doses of WELCHOL may cause more severe local gastrointestinal effects (e.g., constipation).
5DESCRIPTION
WELCHOL (colesevelam hydrochloride) is a non-absorbed, polymeric, lipid-lowering and glucose-lowering agent for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding molecule.
Colesevelam hydrochloride is poly(allylamine hydrochloride) cross-linked with epichlorohydrin and alkylated with 1-bromodecane and (6-bromohexyl)-trimethylammonium bromide. The chemical name (IUPAC) of colesevelam hydrochloride is allylamine polymer with 1-chloro-2,3-epoxypropane, [6-(allylamino)-hexyl]trimethylammonium chloride and N-allyldecylamine, hydrochloride. The chemical structure of colesevelam hydrochloride is represented by the following formula:
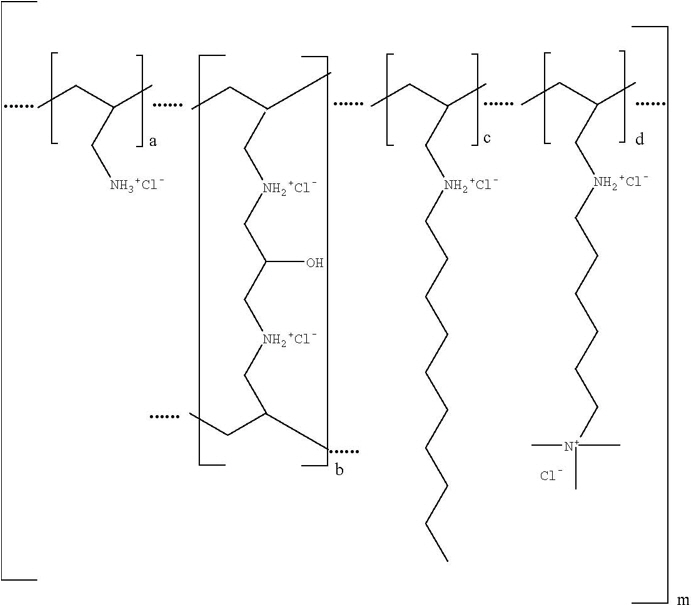
wherein (a) represents allyl amine monomer units that have not been alkylated by either of the 1-bromodecane or (6-bromohexyl)-trimethylammonium bromide alkylating agents or cross-linked by epichlorohydrin; (b) represents allyl amine units that have undergone cross-linking with epichlorohydrin; (c) represents allyl amine units that have been alkylated with a decyl group; (d) represents allyl amine units that have been alkylated with a (6-trimethylammonium) hexyl group, and m represents a number ≥100 to indicate an extended polymer network. A small amount of the amines are dialkylated and are not depicted in the formula above. No regular order of the groups is implied by the structure; cross-linking and alkylation are expected to occur randomly along the polymer chains. A large amount of the amines are protonated. The polymer is depicted in the hydrochloride form; a small amount of the halides are bromide. Colesevelam hydrochloride is hydrophilic and insoluble in water.
WELCHOL tablets are off-white, oval, film-coated, solid tablets each containing 625 mg colesevelam hydrochloride. In addition, each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, silicon dioxide, HPMC (hydroxypropyl methylcellulose), and acetylated monoglyceride. The tablets are imprinted using a water-soluble black ink (<5 calories per 6 tablets).
WELCHOL for oral suspension is a citrus-flavored, white to pale yellow powder containing yellow granules packaged in a packet containing 3.75 gram colesevelam hydrochloride. In addition, each packet contains the following inactive ingredients: lemon flavor, orange flavor, propylene glycol alginate, simethicone, aspartame, citric acid, medium chain triglycerides, and magnesium trisilicate (<5 calories per 3.75 gram single-dose packet). PHENYLKETONURICS: WELCHOL for oral suspension contains 27 mg phenylalanine per 3.75 gram dose.
6HOW SUPPLIED/STORAGE AND HANDLING
WELCHOL 625 mg tablets are supplied as off-white, solid tablets imprinted with the word "Sankyo" and "C01" on one side and are available as follows:
- Bottles of 180 – NDC 65597-701-18
WELCHOL 3.75 gram packets for oral suspension contain a white to pale yellow powder containing yellow granules and are available as follows:
- Cartons of 30 packets – NDC 65597-902-30
7PRINCIPAL DISPLAY PANEL
NDC 65597-701-18

8PRINCIPAL DISPLAY PANEL - 3.75 g Packet Label
TEAR HERE
Sugar-Free
This packet is contained within the
Welchol™
3.75 g
Citrus Flavor
Each packet contains 3.75 grams of
Rx only
Keep Out of Reach of Children.

9PRINCIPAL DISPLAY PANEL - 3.75 g Packet Carton
Keep Out of Reach of Children.
NDC 65597-902-30
30 packets
Welchol™
3.75 g
Each packet contains 3.75 grams of colesevelam hydrochloride.
Rx only
Dosing and use: see package insert.

10PRINCIPAL DISPLAY PANEL - 3.75 g Bar Package Carton - 209-30
NDC 65587-209-30
Welchol
3.75 g
30 CHEWABLE BARS
RX ONLY
EACH BAR CONTAINS 3.75 g
CHILD-RESISTANT WRAPPERS
CONTAINS MEDICATION
DOSING AND USE:
NOTE TO PHARMACISTS:
CHOCOLATE FLAVOR

11PRINCIPAL DISPLAY PANEL - 3.75 g Bar Package Carton - 210-30
NDC 65587-210-30
Welchol
3.75 g
30 CHEWABLE BARS
RX ONLY
EACH BAR CONTAINS 3.75 g
CHILD-RESISTANT WRAPPERS
CONTAINS MEDICATION
DOSING AND USE:
NOTE TO PHARMACISTS:
STRAWBERRY FLAVOR

12PRINCIPAL DISPLAY PANEL - 3.75 g Bar Package Carton - 208-30
NDC 65587-208-30
Welchol
3.75 g
30 CHEWABLE BARS
RX ONLY
EACH BAR CONTAINS 3.75 g
CHILD-RESISTANT WRAPPERS
CONTAINS MEDICATION
DOSING AND USE:
NOTE TO PHARMACISTS:
CARAMEL FLAVOR




