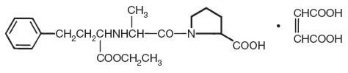
Enalapril
What is Vasotec (Enalapril)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The aim of this cross-over trial is to assess aliskiren, a direct renin inhibitor, as a novel treatment to block complement activation in the kidneys and thereby attenuate renal disease and stabilize or improve kidney function and compare it to the currently used treatment with the angiotensin converting enzyme inhibitor, enalapril, in patients with the complement-mediated renal disease C3 glomeru...
Summary: Changes to gastric pH, gastric emptying time, gastrointestinal transit-time or the pre-systemic metabolizing effect of enzymes secreted in the mucosa may all alter the pharmacokinetics of medicines. These factors are potentially influenced by bariatric surgery. Little is so far known about how gastric bypass and sleeve gastrectomy impacts the biological availability of medication. In this study th...
Summary: The goal of this clinical trial is to explore if enalapril can be used to treat painful venous malformations. The main question it aims to answer are: \- Can enalapril reduce pain and volume of the malformation and increase quality of life in patients with painful venous malformations? Participants will: Receive a dose of enalapril 5 mg once daily. If it doesn't have effect the dose of enalapril w...
Related Latest Advances
Brand Information
- When pregnancy is detected, discontinue VASOTEC
- Drugs that act directly on the renin-angiotensin system can cause injury and death to thedeveloping fetus. (SeeWARNINGS, Fetal Toxicity.)
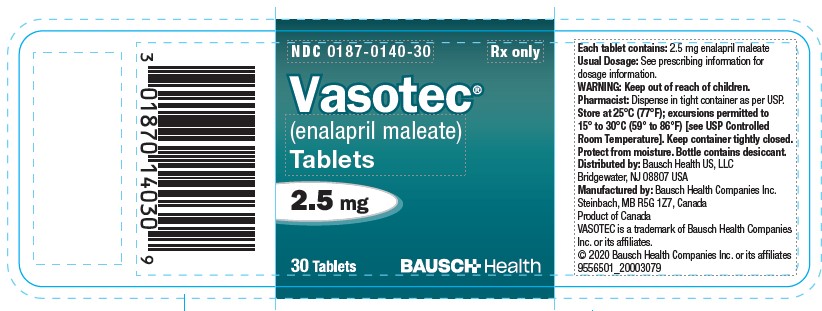
(enalapril maleate)
Tablets
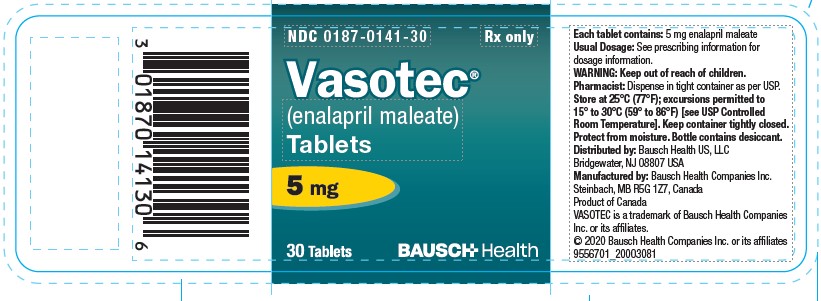
(enalapril maleate)
Tablets
(enalapril maleate)
Tablets

(enalapril maleate)
Tablets



