Brand Name
Sensipar
Generic Name
Cinacalcet
View Brand Information FDA approval date: April 04, 2004
Classification: Calcium-sensing Receptor Agonist
Form: Tablet
What is Sensipar (Cinacalcet)?
Cinacalcet hydrochloride is a calcium-sensing receptor agonist indicated for: Secondary Hyperparathyroidism in adult patients with chronic kidney disease on dialysis.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Sensipar (cinacalcet hydrochloride)
1DOSAGE FORMS AND STRENGTHS
Sensipar is available as film-coated tablets.
Sensipar tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “30” or “60” or “90” on the opposite side of the 30 mg, 60 mg, or 90 mg strengths, respectively.
2CONTRAINDICATIONS
Sensipar treatment initiation is contraindicated if serum calcium is less than the lower limit of the normal range
3ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
- Hypocalcemia
- Upper Gastrointestinal Bleeding
- Hypotension, Worsening Heart Failure and/or Arrhythmias
- Adynamic Bone Disease
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Secondary Hyperparathyroidism in Patients withChronic Kidney Disease on Dialysis
In three double-blind, placebo-controlled clinical trials, 1126 patients with CKD on dialysis received study drug (656 Sensipar, 470 placebo) for up to 6 months. The most frequently reported adverse reactions are listed in Table 1.
Seizures were observed in 1.4% (13/910) of Sensipar-treated patients and 0.7% (5/641) of placebo-treated patients across all completed placebo-controlled trials.
In a randomized, double-blind placebo-controlled study of 3883 patients with secondary HPT and CKD receiving dialysis in which patients were treated for up to 64 months (mean duration of treatment was 21 months in the Sensipar group), the most frequently reported adverse reactions (incidence of ≥ 5% in the Sensipar group and a difference ≥ 1% compared to placebo) are listed in Table 2.
Additional adverse reaction rates from the long-term, randomized, double-blind placebo-controlled study for Sensipar versus placebo are as follows: seizure (2.5%, 1.6%), rash (2.2%, 1.9%), hypersensitivity reactions (9.4%, 8.3%).
Patients with Parathyroid Carcinoma and Primary Hyperparathyroidism
The safety profile of Sensipar in these patient populations is generally consistent with that seen in patients with CKD on dialysis. Forty six patients were treated with Sensipar in a single-arm study, 29 with Parathyroid Carcinoma and 17 with intractable pHPT. Nine (20%) of the patients withdrew from the study due to adverse events. The most frequent adverse reactions and the most frequent cause of withdrawal in these patient populations were nausea and vomiting. Severe or prolonged cases of nausea and vomiting can lead to dehydration and worsening hypercalcemia so careful monitoring of electrolytes is recommended in patients with these symptoms.
Eight patients died during treatment with Sensipar in this study, 7 with Parathyroid Carcinoma (24%) and 1 (6%) with intractable pHPT. Causes of death were cardiovascular (5 patients), multi-organ failure (1 patient), gastrointestinal hemorrhage (1 patient) and metastatic carcinoma (1 patient). Adverse events of hypocalcemia were reported in three patients (7%).
Seizures were observed in 0.7% (1/140) of cinacalcet-treated patients and 0.0% (0/46) of placebo-treated patients in all clinical studies.
In a randomized double-blind, placebo-controlled study of 67 patients with primary hyperparathyroidism for whom parathyroidectomy would be indicated on the basis of serum calcium levels, but who are unable to undergo surgery, the most common adverse reactions are listed in Table 4.
Table 4. Adverse Reactions Occurring in ≥10% of Subjects in a Double-Blind, Placebo-Controlled Study in Patients with Primary Hyperparathyroidism
Hypocalcemia
In 26-week studies of patients with secondary HPT and CKD on dialysis 66% of patients receiving Sensipar compared with 25% of patients receiving placebo developed at least one serum calcium value less than 8.4 mg/dL, whereas, 29% of patients receiving Sensipar compared with 11% of patients receiving placebo developed at least one serum calcium value less than 7.5 mg/dL. Less than 1% of patients in each group permanently discontinued study drug due to hypocalcemia.
In a randomized, double-blind, placebo-controlled study in patients with secondary HPT and CKD receiving dialysis in which patients were treated for up to 64 months (mean duration of treatment was 21 months in the cinacalcet group), 75% of patients receiving Sensipar compared with 29% of patients receiving placebo developed at least one serum calcium value less than 8.4 mg/dL and 33% of cinacalcet patients compared with 12% of patients receiving placebo had at least one serum calcium value less than 7.5 mg/dL. Most of the cases of severe hypocalcemia less than 7.5 mg/dL (21/33 = 64%) occurred during the first 6 months. In this trial, 1.1% of patients receiving Sensipar and 0.1% of patients receiving placebo permanently discontinued study drug due to hypocalcemia.
During a placebo-controlled part of a 52-week study in patients with primary HPT who met criteria for parathyroidectomy on the basis of corrected total serum calcium (> 11.3 mg/dL [2.82 mmol/L] and ≤ 12.5 mg/dL [3.12 mmol/L]), serum calcium less than 8.4 mg/dL was observed in 6.1% (2/33) of Sensipar-treated patients and 0% (0/34) of placebo-treated patients.
3.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of Sensipar. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Rash and hypersensitivity reactions (including angioedema and urticaria), and myalgia
- Isolated, idiosyncratic cases of hypotension, worsening heart failure, and/or arrhythmia have been reported in patients with impaired cardiac function
- Gastrointestinal bleeding
- Chondrocalcinosis pyrophosphate (acute pseudogout)
4OVERDOSAGE
Overdosage of Sensipar may lead to hypocalcemia. In the event of overdosage, patients should be monitored for signs and symptoms of hypocalcemia and appropriate measures taken to correct serum calcium levels
Since Sensipar is highly protein bound, hemodialysis is not an effective treatment for overdosage of Sensipar.
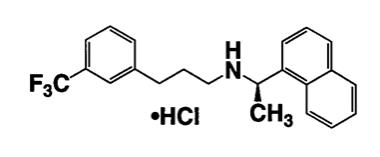
5DESCRIPTION
Sensipar tablets contain the hydrochloride salt of the active ingredient cinacalcet, a positive modulator of the calcium sensing receptor . The empirical formula for cinacalcet is C
Sensipar tablets are formulated as light-green, film-coated, oval-shaped tablets for oral administration in strengths of 30 mg, 60 mg, and 90 mg of cinacalcet as the free base equivalent (33 mg, 66 mg, and 99 mg as the hydrochloride salt, respectively).
Inactive Ingredients
The following are the inactive ingredients in Sensipar tablets: pre-gelatinized starch, microcrystalline cellulose, povidone, crospovidone, colloidal silicon dioxide and magnesium stearate. Tablets are coated with color (Opadry
6HOW SUPPLIED/STORAGE AND HANDLING
Sensipar 30 mg tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “30” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-073-30)
Sensipar 60 mg tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “60” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-074-30)
Sensipar 90 mg tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “90” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-075-30)
Storage
Store at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F). [See USP controlled room temperature].
7PATIENT COUNSELING INFORMATION
- Hypocalcemia: Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions (5.1)].
- Upper Gastrointestinal Bleeding: Advise patients to report any symptoms of upper gastrointestinal bleeding to their health care provider [see Warnings and Precautions (5.2)].
- Heart Failure: Advise patients with heart failure that use of Sensipar may worsen their heart failure and additional monitoring may be required [see Warnings and Precautions (5.3)].
- Advise patients to report nausea and vomiting to their health care provider
- Advise patients to take Sensipar with food or shortly after a meal and to take the tablets whole and not divide them
- Inform patients of the importance of regular blood tests, in order to monitor the safety and efficacy of Sensipar therapy.
Sensipar
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
Patent: http://pat.amgen.com/sensipar/
© 2004-2019 Amgen Inc. All rights reserved.
www.sensipar.com
1-800-77-AMGEN (1-800-772-6436)
1xxxxxx – v14
8PRINCIPAL DISPLAY PANEL
AMGEN
NDC 55513-073-30
Sensipar
(cinacalcet) Tablets
Rx Only
30 tablets
30 mg
Each tablet contains:
Cinacalcet 30 mg (equivalent to 33 mg of cinacalcet hydrochloride).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
See USP controlled room temperature.
Dispense in tight, light-resistant container per USP.
Dosage : See Package Insert.
© 2004-2018 Amgen Inc. All rights reserved.
Patent : http://pat.amgen.com/sensipar/
Distributed by : Amgen,
One Amgen Center Drive,
Thousand Oaks, CA 91320-1799
Made in Japan

9PRINCIPAL DISPLAY PANEL
AMGEN
NDC 55513-074-30
Sensipar
(cinacalcet) Tablets
Rx Only
30 tablets
60 mg
Each tablet contains:
Cinacalcet 60 mg (equivalent to 66 mg of cinacalcet hydrochloride).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
See USP controlled room temperature.
Dispense in tight, light-resistant container per USP.
Dosage : See Package Insert.
© 2004-2018 Amgen Inc. All rights reserved.
Patent : http://pat.amgen.com/sensipar/
Distributed by : Amgen,
One Amgen Center Drive,
Thousand Oaks, CA 91320-1799
Made in Japan

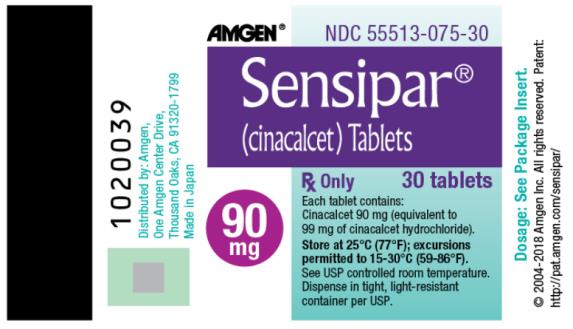
10PRINCIPAL DISPLAY PANEL
AMGEN
NDC 55513-075-30
Sensipar
(cinacalcet) Tablets
Rx Only
30 tablets
90 mg
Each tablet contains:
Cinacalcet 90 mg (equivalent to 99 mg of cinacalcet hydrochloride).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
See USP controlled room temperature.
Dispense in tight, light-resistant container per USP.
Dosage : See Package Insert.
© 2004-2018 Amgen Inc. All rights reserved.
Patent : http://pat.amgen.com/sensipar/
Distributed by : Amgen,
One Amgen Center Drive,
Thousand Oaks, CA 91320-1799
Made in Japan