Generic Name
Diltiazem
Brand Names
Matzim, Tiadylt, Cardizem, Diltiazem HCI, Cartia XT, Tiazac
FDA approval date: November 05, 1992
Classification: Calcium Channel Blocker
Form: Injection, Tablet, Capsule
What is Matzim (Diltiazem)?
Diltiazem Hydrochloride Extended-Release Tablet is a nondihydropyridine calcium channel blocker indicated for: treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. It can be used alone or in combination with other antihypertensives.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Matzim LA (Diltiazem Hydrochloride)
1DOSAGE AND ADMINISTRATION
Take Matzim LA (diltiazem hydrochloride) extended-release tablets once a day at approximately the same time. Do not chew or crush the tablet.
1.1Hypertension
Initiate dosing at 180 to 240 mg once daily, although some patients may respond to lower doses. Titrate according to blood pressure to a maximum of 540 mg daily. Maximum antihypertensive effect is usually observed by 14 days of chronic therapy.
1.2Angina
Initiate dosing at 180 mg once daily and increase dose at intervals of 7 to 14 days if adequate response is not obtained, to a maximum of 360 mg.
1.3Switching to Matzim LA (Diltiazem Hydrochloride)Extended-Release Tablets
Patients controlled on diltiazem alone or in combination with other medications may be switched to diltiazem hydrochloride extended-release tablets once a day at the nearest equivalent total daily dose. Higher doses of Matzim LA (diltiazem hydrochloride) extended-release tablets may be needed in some patients based on clinical response.
2DOSAGE FORMS AND STRENGTHS
Extended-release tablets with 180 mg, 240 mg, 300 mg, 360 mg, or 420 mg diltiazem hydrochloride per tablet.
Matzim LA (diltiazem hydrochloride) extended-release tablets, 180 mg are supplied as white, capsule-shaped tablets debossed with “
Matzim LA (diltiazem hydrochloride) extended-release tablets, 240 mg are supplied as white, capsule-shaped tablets debossed with “
Matzim LA (diltiazem hydrochloride) extended-release tablets, 300 mg are supplied as white, capsule-shaped tablets debossed with “
Matzim LA (diltiazem hydrochloride) extended-release tablets, 360 mg are supplied as white, capsule-shaped tablets debossed with “
Matzim LA (diltiazem hydrochloride) extended-release tablets, 420 mg are supplied as white, capsule-shaped tablets debossed with “
3CONTRAINDICATIONS
Matzim LA (diltiazem hydrochloride) extended-release tablets are contraindicated in:
Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker. Patients with second- or third-degree AV block except in the presence of a functioning ventricular pacemaker. Patients with hypotension (less than 90 mm Hg systolic). Patients who have demonstrated hypersensitivity to the drug. Patients with acute myocardial infarction and pulmonary.
4ADVERSE REACTIONS
The following adverse reactions are described in greater detail, in other sections:
- Bradycardia and AV block
- Heart failure
- Acute hepatic injury
- Severe skin reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
For the hypertension studies, the following table presents adverse reactions more common on diltiazem than on placebo (but excluding events with no plausible relationship to treatment), as reported in placebo-controlled hypertension trials in patients receiving a diltiazem hydrochloride extended-release formulation (once-a-day dosing) up to 540 mg.
In the angina study, the adverse event profile of diltiazem hydrochloride extended-release tablets was consistent with what has been previously described for diltiazem hydrochloride extended-release tablets and other formulations of diltiazem HCl. The most frequent adverse effects experienced by diltiazem hydrochloride extended-release tablets-treated patients were edema lower-limb (6.8%), dizziness (6.4%), fatigue (4.8%), bradycardia (3.6%), first-degree atrioventricular block (3.2%), and cough (2%).
In addition, the following events have been reported infrequently (less than 1%) in angina or hypertension trials:
Cardiovascular: Angina, bundle branch block, palpitations, syncope, tachycardia, ventricular extrasystoles [see .
Nervous System: Abnormal dreams, amnesia, depression, gait abnormality, hallucinations, insomnia, nervousness, paresthesia, personality change, somnolence, tinnitus, tremor.
Gastrointestinal: Anorexia, constipation, diarrhea, dry mouth, dysgeusia, dyspepsia, thirst, vomiting, weight increase.
Dermatological: Petechiae, photosensitivity, pruritus, urticaria [see .
Other: Amblyopia, CPK increase, dyspnea, epistaxis, eye irritation, hyperglycemia, hyperuricemia, impotence, muscle cramps, nasal congestion, nocturia, osteoarticular pain, polyuria, sexual difficulties.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of diltiazem. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing reactions have been reported infrequently in patients receiving diltiazem: acute generalized exanthematous pustulosis, allergic reactions, alopecia, angioedema (including facial or periorbital edema), erythema multiforme, extrapyramidal symptoms, gingival hyperplasia, hemolytic anemia, increased bleeding time, leukopenia, photosensitivity (including lichenoid keratosis and hyperpigmentation at sun-exposed skin areas), purpura, retinopathy, myopathy, and thrombocytopenia.
In addition, events such as myocardial infarction have been observed which are not readily distinguishable from the natural history of the disease in these patients.
A number of well-documented cases of generalized rash, some characterized as leukocytoclastic vasculitis, have been reported.
5OVERDOSAGE
The oral LD
The toxic dose in man is not known. Blood levels after a standard dose of diltiazem can vary over tenfold, limiting the usefulness of blood levels in overdose cases.
There have been 29 reports of diltiazem overdose in doses ranging from less than 1 g to 18 g. Sixteen of these reports involved multiple drug ingestions.
Twenty-two reports indicated patients had recovered from diltiazem overdose ranging from less than 1 g to 10.8 g. There were seven reports with a fatal outcome; although the amount of diltiazem ingested was unknown, multiple drug ingestions were confirmed in six of the seven reports.
Events observed following diltiazem overdose included bradycardia, hypotension, heart block, and cardiac failure. Most reports of overdose described some supportive medical measure and/or drug treatment. Bradycardia frequently responded favorably to atropine as did heart block, although cardiac pacing was also frequently utilized to treat heart block. Fluids and vasopressors were used to maintain blood pressure and in cases of cardiac failure, inotropic agents were administered. In addition, some patients received treatment with ventilatory support, gastric lavage, activated charcoal, and/or intravenous calcium.
In the event of overdose or exaggerated response, institute appropriate supportive measures and gastrointestinal decontamination. Diltiazem does not appear to be removed by peritoneal or hemodialysis. Limited data suggest that plasmapheresis or charcoal hemoperfusion may hasten diltiazem elimination following overdose. Based on the known pharmacological effects of diltiazem and/or reported clinical experiences, the following measures may be considered:
Bradycardia: Administer atropine (0.60 to 1.0 mg). If there is no response to vagal blockage, administer isoproterenol cautiously.
High-degree AV Block: Treat as for bradycardia above. Fixed high-degree AV block should be treated with cardiac pacing.
Cardiac Failure: Administer inotropic agents (isoproterenol, dopamine, or dobutamine) and diuretics.
Hypotension: Use vasopressors (e.g., dopamine or norepinephrine).
Actual treatment and dosage should depend on the severity of the clinical situation and the judgment and experience of the treating physician.
6DESCRIPTION
Matzim
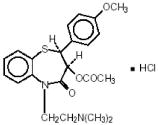
Diltiazem hydrochloride, USP is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol and chloroform. It has a molecular weight of 450.99. Matzim LA (Diltiazem Hydrochloride) Extended-Release Tablets, for oral administration, are formulated as a once-a-day extended-release tablet containing 180 mg, 240 mg, 300 mg, 360 mg or 420 mg of diltiazem hydrochloride.
Also contains: candelilla wax powder, colloidal silicon dioxide, corn starch, ethyl acrylate and methyl methacrylate copolymer dispersion, ethylcellulose, hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, nonoxynol 100, polyethylene oxide, polysorbate 80, povidone, sucrose, talc, titanium dioxide and triacetin.
7HOW SUPPLIED/STORAGE AND HANDLING
Matzim
180 mg - White, capsule-shaped tablets debossed with “
Bottles of 30 NDC 52544-691-30
Bottles of 90 NDC 52544-691-19
Bottles of 30 NDC 52544-692-30
Bottles of 90 NDC 52544-692-19
300 mg - White, capsule-shaped tablets debossed with “
Bottles of 30 NDC 52544-693-30
Bottles of 90 NDC 52544-693-19
360 mg - White, capsule-shaped tablets debossed with “
Bottles of 30 NDC 52544-694-30
Bottles of 90 NDC 52544-694-19
420 mg - White, capsule-shaped tablets debossed with “
Bottles of 30 NDC 52544-695-30
Bottles of 90 NDC 52544-695-19
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Avoid excessive humidity and temperatures above 30°C (86°F).
Dispense in tight, light resistant container as defined in USP.
8PATIENT COUNSELING INFORMATION
Advise patients:
- That the Matzim LA (diltiazem hydrochloride) extended-release tablet should be swallowed whole and not chewed or crushed.
- To consult the physician who prescribed Matzim LA (diltiazem hydrochloride) extended-release tablets before taking or stopping any other medications, including over-the-counter products or nutritional supplements, such as St. John’s wort.
- To contact the physician who prescribed Matzim LA (diltiazem hydrochloride) extended-release tablets or any other physician immediately if they experience possible adverse reactions, including bradycardia, arrhythmias, symptoms indicative of hypotension or heart failure, hepatic and skin reactions.
- To consult their physician if they become pregnant while taking Matzim LA (diltiazem hydrochloride) extended-release tablets or plan to become pregnant.
Manufactured For:
Rev. D 5/2025
9PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 180 MG
NDC 52544
10PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 240 MG
NDC 52544
11PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 300 MG
NDC 52544
12PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 360 MG
NDC 52544
13PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 420 MG
NDC 52544