Brand Name
Crenessity
Generic Name
Crinecerfont
View Brand Information FDA approval date: December 10, 2024
Form: Capsule, Solution
What is Crenessity (Crinecerfont)?
CRENESSITY is indicated as adjunctive treatment to glucocorticoid replacement to control androgens in adults and pediatric patients 4 years of age and older with classic congenital adrenal hyperplasia . CRENESSITY is a corticotropin-releasing factor type 1 receptor antagonist indicated as adjunctive treatment to glucocorticoid replacement to control androgens in adults and pediatric patients 4 years of age and older with classic congenital adrenal hyperplasia .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 2, Open-Label Study to Evaluate the Pharmacokinetics, Safety, Tolerability, and Pharmacodynamics of Crinecerfont in Pediatric Subjects 0 to <2 Years of Age With Congenital Adrenal Hyperplasia
Summary: The main objective for this study is to evaluate the pharmacokinetics (PK) of crinecerfont in pediatric participants 0 to \<2 years of age with congenital adrenal hyperplasia (CAH).
Brand Information
Crenessity (crinecerfont)
1INDICATIONS AND USAGE
CRENESSITY is indicated as adjunctive treatment to glucocorticoid replacement to control androgens in adults and pediatric patients 4 years of age and older with classic congenital adrenal hyperplasia (CAH)
2DOSAGE FORMS AND STRENGTHS
CRENESSITY is available as:
- Capsules
- Oral Solution: 50 mg/mL in light yellow to orange, clear to slightly opalescent oral solution
3CONTRAINDICATIONS
CRENESSITY is contraindicated in patients with hypersensitivity to crinecerfont or any excipients of CRENESSITY. Reactions have included throat tightness, angioedema, and generalized rash
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions
- Risk of Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid Therapy
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults with Congenital Adrenal Hyperplasia (CAH)
The safety of CRENESSITY in adults was assessed in Study 1, a randomized, double-blind, placebo-controlled study of 182 adults aged 18 to 58 years with classic CAH due to 21-hydroxylase deficiency. A total of 122 subjects received CRENESSITY 100 mg twice daily and 59 subjects received placebo twice daily for up to 24 weeks
Adverse Reactions Leading to Discontinuation of Treatment
A total of 3% of CRENESSITY-treated subjects and no placebo-treated subjects discontinued treatment because of adverse reactions of restlessness, apathy, dyspepsia, nausea, and vomiting.
Commonly Observed Adverse Reactions
Adverse reactions that occurred in ≥4% of CRENESSITY-treated subjects and more frequently than in placebo-treated subjects are presented in
Table 4:Adverse Reactions (≥4%) in Adults with Congenital Adrenal HyperplasiaTreated withCRENESSITY and Occurring More Frequently Than in Placebo-Treated Subjects
Suicidal Ideation and Behavior
Study 1 excluded subjects with active suicidal ideation with intent or plan within the six months prior to screening and those with a history of suicidal behavior within the past year, based on the Columbia-Suicide Severity Rating Scale (C-SSRS) administered at screening. The C-SSRS was administered to subjects at regular intervals during the study. Three of 122 (2.5%) CRENESSITY-treated subjects reported suicidal ideation without method, intent or plan on the C-SSRS during the 24-week double-blind treatment period compared to 1 of 59 (1.7%) placebo-treated subjects. One of the three subjects receiving CRENESSITY and the placebo-treated subject reported a lifetime history of suicidal ideation.
One CRENESSITY-treated subject without a history of suicidal ideation or behavior attempted suicide during the open-label period after 320 days of treatment.
Laboratory Findings
Neutrophil count less than 2 x 10
Pediatric Patients with Congenital Adrenal Hyperplasia
The safety of CRENESSITY in pediatric patients was evaluated in Study 2, a randomized, double-blind placebo-controlled study of 103 pediatric subjects aged 4 to 17 years with classic CAH due to 21-hydroxylase deficiency. Pediatric subjects were randomized to receive CRENESSITY twice daily (N=69) or placebo (N=34) for 28 weeks, using weight-based dosing (50 mg twice daily via oral solution for subjects 20 to <55 kg, or 100 mg twice daily via oral capsules for subjects ≥55 kg)
Adverse Reactions Leading to Discontinuation of Treatment
A total of 3% of CRENESSITY-treated subjects and no placebo-treated subjects discontinued treatment because of adverse reactions of abdominal pain, myalgia, and dizziness.
Commonly Observed Adverse Reactions
Adverse reactions that occurred at an incidence of ≥4% in CRENESSITY-treated pediatric subjects (50 mg twice daily or 100 mg twice daily) and greater than placebo are presented in
Table 5:Adverse Reactions (≥ 4%) in Pediatric Subjectswith Congenital Adrenal Hyperplasia Treatedwith CRENESSITY and Occurring More Frequently Than in Placebo-Treated Subjects
1Abdominal pain includes: abdominal pain, abdominal pain upper and abdominal pain lower
Suicidal Ideation and Behavior
Study 2 excluded subjects with active suicidal ideation with intent or plan within six months prior to screening or those with a lifetime history of suicidal behavior based on the C-SSRS administered at screening. Four of 67 (6%) CRENESSITY-treated subjects and 0 of the 31 (0%) placebo-treated subjects reported suicidal ideation without method, intent or plan on the C-SSRS during the 28-week double-blind treatment period. Two of the four CRENESSITY-treated subjects reported a lifetime history of suicidal ideation. There were no completed suicides or suicide attempts.
Laboratory Findings
Neutrophil count less than 2 x 10
5DESCRIPTION
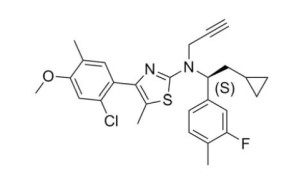
CRENESSITY contains crinecerfont, a selective corticotropin-releasing factor type 1 receptor antagonist, present as crinecerfont free base, with the chemical name, 2-thiazolamine, 4-(2-chloro-4-methoxy-5-methylphenyl)-
CRENESSITY Capsules
CRENESSITY capsules are intended for oral administration only. Each capsule contains 25 mg, 50 mg, or 100 mg of crinecerfont free base. Inactive ingredients include lauroyl polyoxyl-32 glycerides, medium chain triglycerides, propylene glycol dicaprylate/dicaprate, and Vitamin E polyethylene glycol succinate. The capsule shell contains gelatin, glycerin, red iron oxide, Sorbitol glycerin blend, titanium dioxide, and yellow iron oxide.
CRENESSITY Oral Solution
The oral solution formulation contains 50 mg/mL of crinecerfont free base. Inactive ingredients include butylated hydroxytoluene, medium-chain triglycerides, oleoyl polyoxyl glycerides, orange flavor, and saccharin.
6PATIENT COUNSELING INFORMATION
Advise the patients and/or caregivers to read the FDA-approved patient labeling (
Administration Information
Counsel patients that CRENESSITY must be taken with a meal, without regard to fat or caloric content
Drug Interactions
Inform patients that the CRENESSITY dosage will need to be increased if they are taking strong or moderate CYP3A4 inducers
Hypersensitivity Reactions
Advise patients to seek prompt medical attention if signs or symptoms of hypersensitivity reactions occur. Advise patients who have had signs or symptoms of systemic hypersensitivity reactions to CRENESSITY that they should not receive CRENESSITY
Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid Therapy
Inform patients that they should continue glucocorticoids when taking CRENESSITY. Counsel patients that any adjustment of glucocorticoid doses should be done under the guidance of their health care provider. Counsel patients about the continued need for stress dose glucocorticoids during times of increased cortisol need (e.g., during illness)
Pregnancy
Advise women who are exposed to CRENESSITY during pregnancy that there is a pregnancy safety study
For information on CRENESSITY, visit www.CRENESSITY.com or call 1-855-CRNSITY
Distributed by: Neurocrine Biosciences, Inc., San Diego, CA 92130, U.S.A.
CRENESSITY is a trademark of Neurocrine Biosciences, Inc.
