Brand Name
Uptravi
Generic Name
Selexipag
View Brand Information FDA approval date: December 21, 2015
Classification: Prostacyclin Receptor Agonist
Form: Injection, Tablet, Kit
What is Uptravi (Selexipag)?
UPTRAVI is a prostacyclin receptor agonist indicated for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization for PAH.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
UPTRAVI (Selexipag)
1DOSAGE FORMS AND STRENGTHS
UPTRAVI is available in the following presentations:
Film-Coated Tablets
- 200 mcg selexipag [Light yellow tablet debossed with 2]
- 400 mcg selexipag [Red tablet debossed with 4]
- 600 mcg selexipag [Light violet tablet debossed with 6]
- 800 mcg selexipag [Green tablet debossed with 8]
- 1000 mcg selexipag [Orange tablet debossed with 10]
- 1200 mcg selexipag [Dark violet tablet debossed with 12]
- 1400 mcg selexipag [Dark yellow tablet debossed with 14]
- 1600 mcg selexipag [Brown tablet debossed with 16]
UPTRAVI for Injection
- 1800 mcg selexipag [Lyophilized powder white to almost white broken cake or powdered material, supplied in a 10 mL single-dose glass vial]
2CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients.
Concomitant use of strong inhibitors of CYP2C8 (e.g., gemfibrozil)
3OVERDOSAGE
Isolated cases of overdose with UPTRAVI tablets up to 3200 mcg were reported. Mild, transient nausea was the only reported consequence. In the event of overdose, supportive measures must be taken as required. Dialysis is unlikely to be effective because selexipag and its active metabolite are highly protein-bound.
4DESCRIPTION
UPTRAVI contains selexipag, a prostacyclin receptor agonist. The chemical name of selexipag is 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-
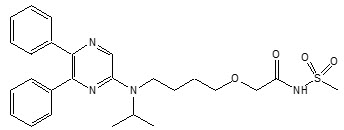
Selexipag is a pale yellow crystalline powder that is practically insoluble in water. In the solid state selexipag is very stable, is not hygroscopic, and is not light sensitive.
UPTRAVI
UPTRAVI
5HOW SUPPLIED/STORAGE AND HANDLING
UPTRAVI
UPTRAVI
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
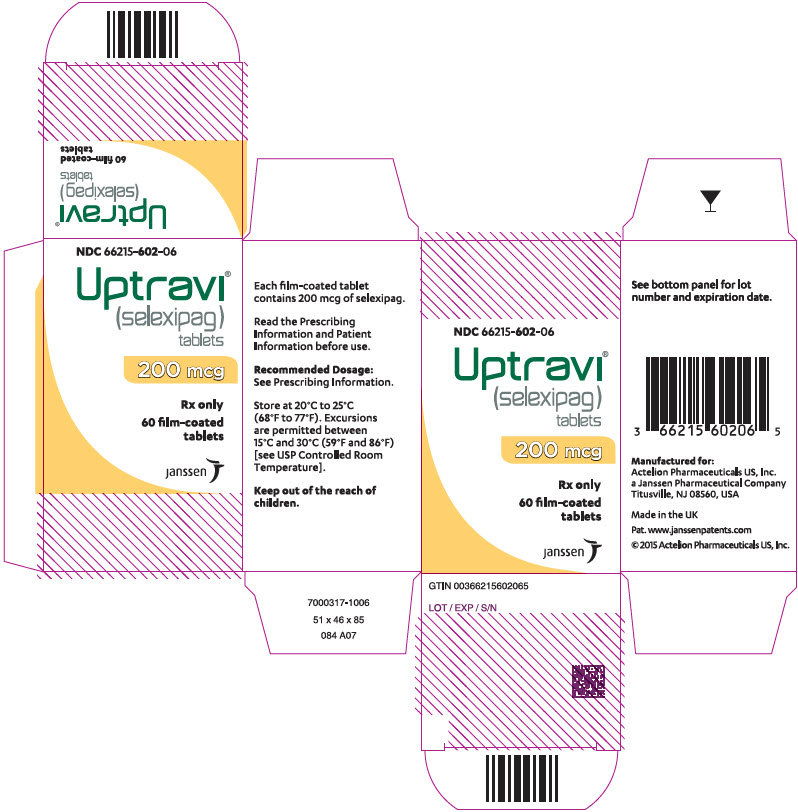
7PRINCIPAL DISPLAY PANEL - 200 mcg Tablet Bottle Carton
NDC 66215-602-06
Uptravi
200 mcg
Rx only
60 film-coated
janssen
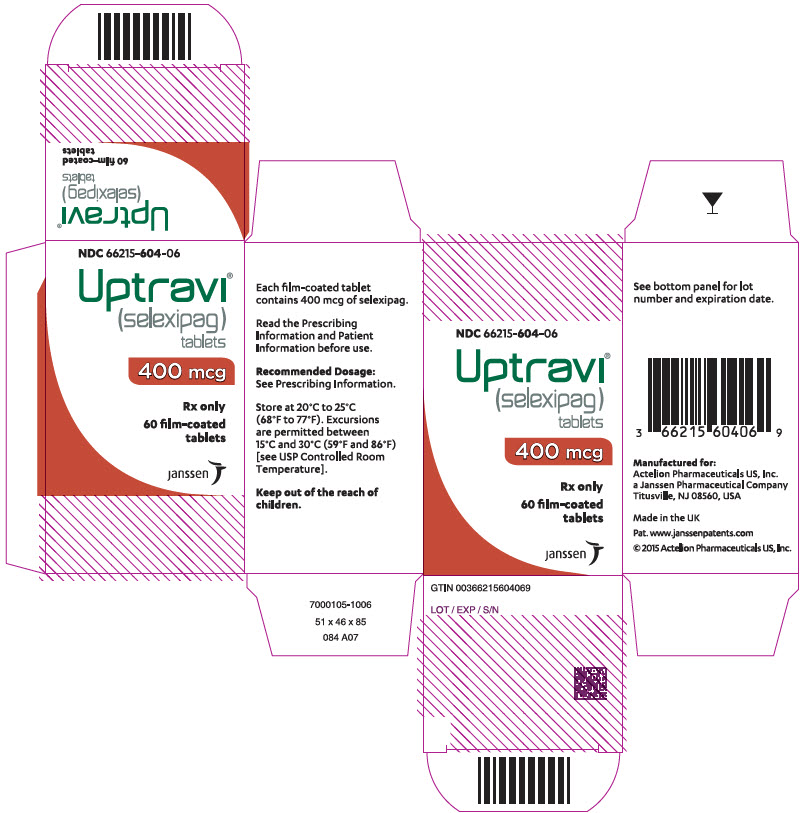
8PRINCIPAL DISPLAY PANEL - 400 mcg Tablet Bottle Carton
NDC 66215-604-06
Uptravi
400 mcg
Rx only
60 film-coated
janssen
9PRINCIPAL DISPLAY PANEL - 600 mcg Tablet Bottle Carton
NDC 66215-606-06
Uptravi
600 mcg
Rx only
60 film-coated
janssen
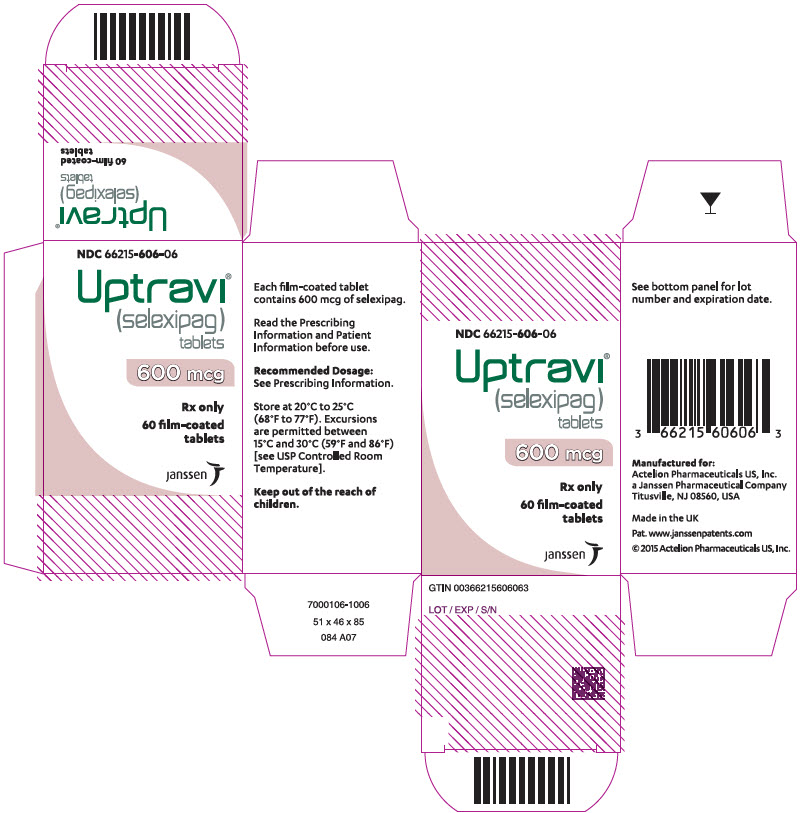
10PRINCIPAL DISPLAY PANEL - 800 mcg Tablet Bottle Carton
NDC 66215-608-06
Uptravi
800 mcg
Rx only
60 film-coated
janssen
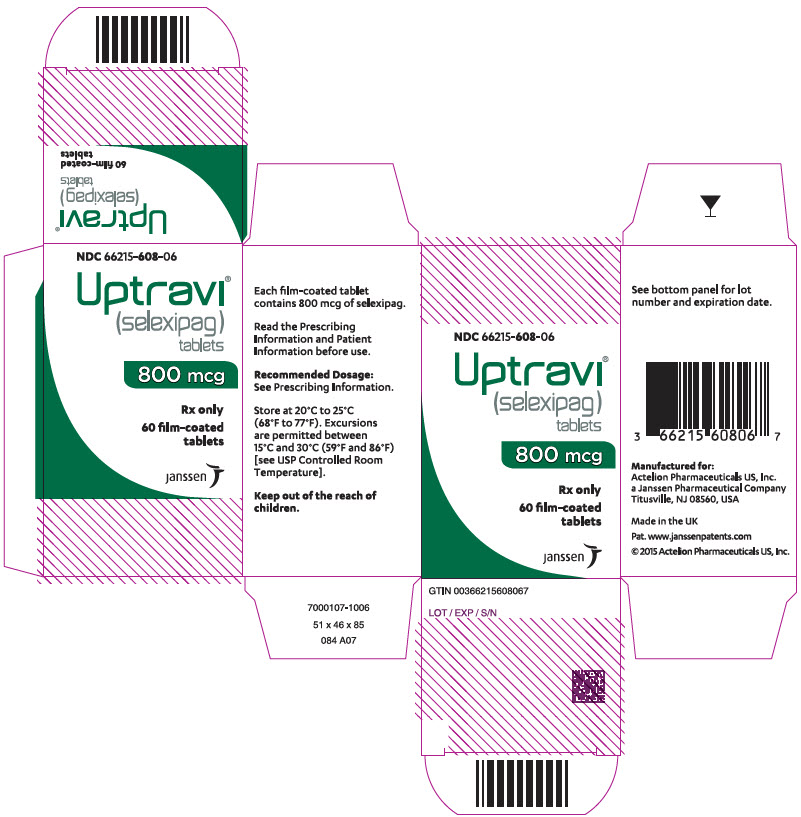
11PRINCIPAL DISPLAY PANEL - 1000 mcg Tablet Bottle Carton
NDC 66215-610-06
Uptravi
1000 mcg
Rx only
60 film-coated
janssen
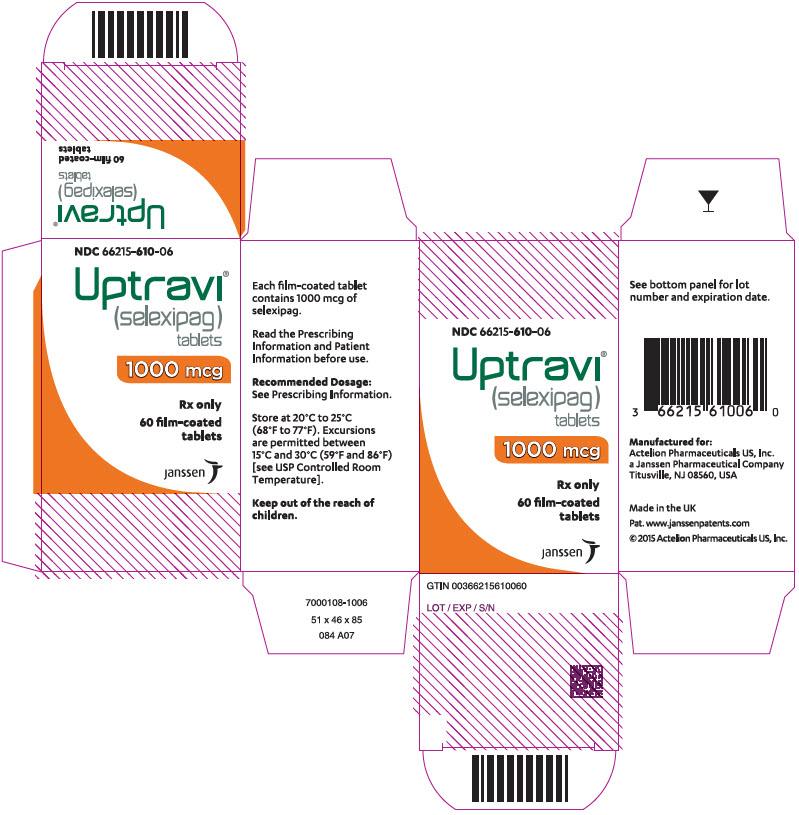
12PRINCIPAL DISPLAY PANEL - 1200 mcg Tablet Bottle Carton
NDC 66215-612-06
Uptravi
1200 mcg
Rx only
60 film-coated
janssen
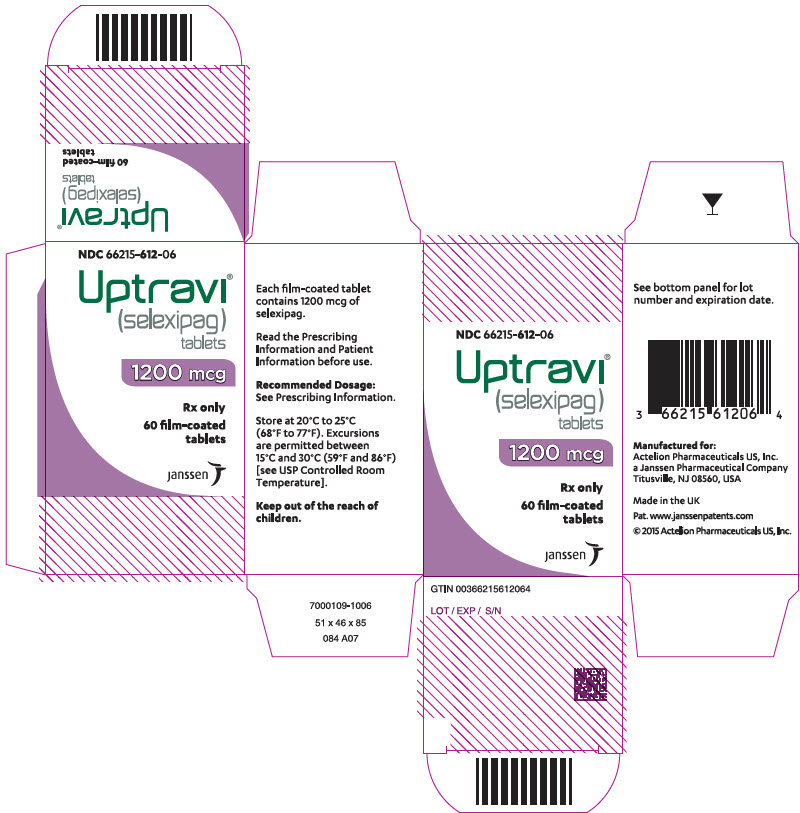
13PRINCIPAL DISPLAY PANEL - 1400 mcg Tablet Bottle Carton
NDC 66215-614-06
Uptravi
1400 mcg
Rx only
60 film-coated
janssen
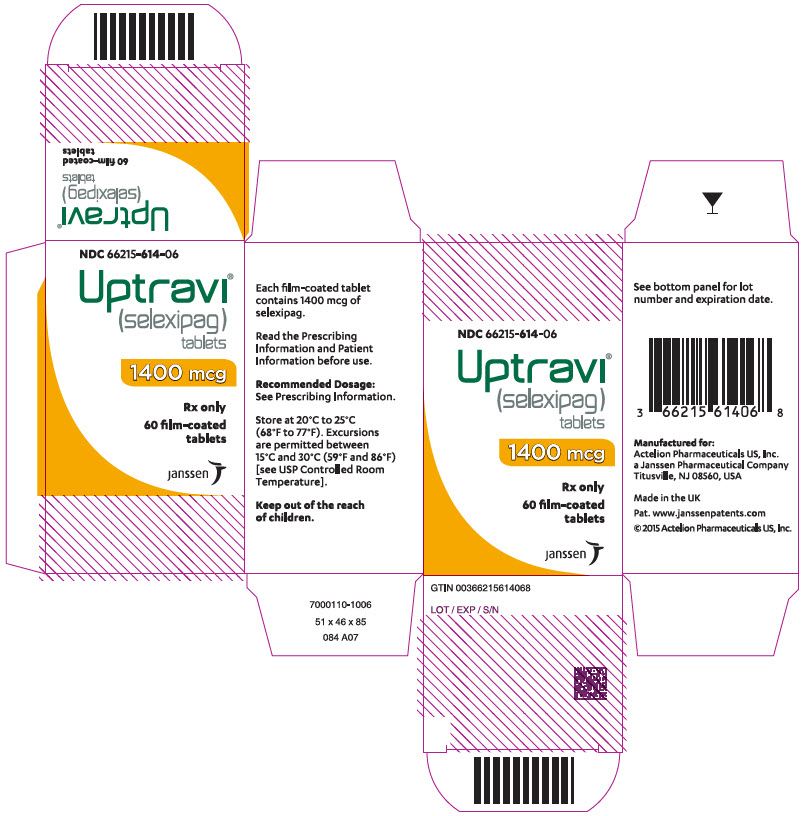
14PRINCIPAL DISPLAY PANEL - 1600 mcg Tablet Bottle Carton
NDC 66215-616-06
Uptravi
1600 mcg
Rx only
60 film-coated
janssen
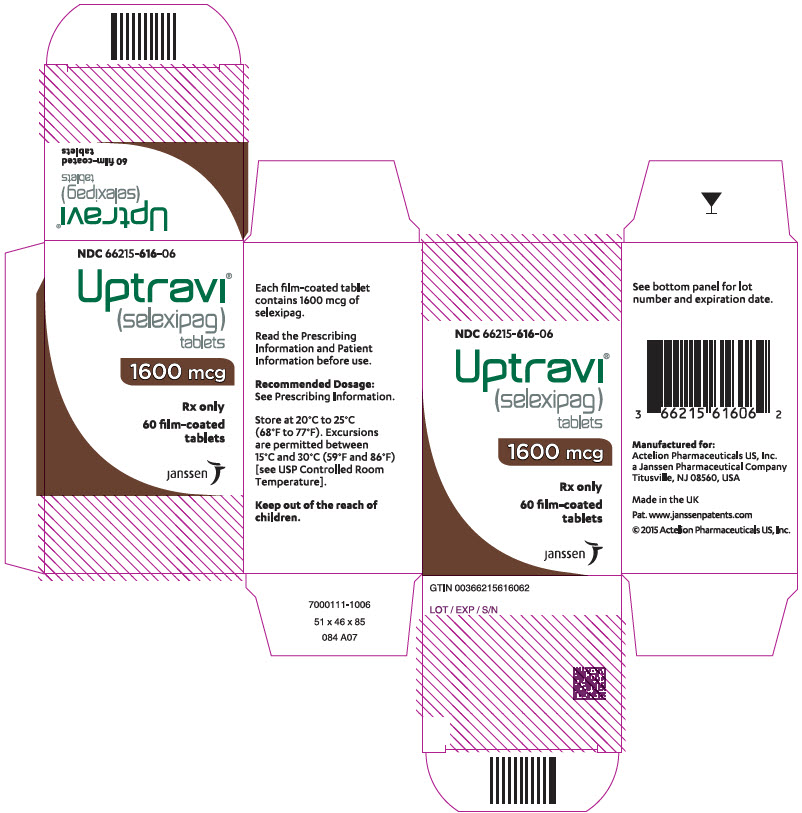
15PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 66215-628-20
TITRATION PACK
Uptravi
200 mcg
Rx only
140 film-coated
Uptravi
800 mcg
Rx only
60 film-coated
janssen
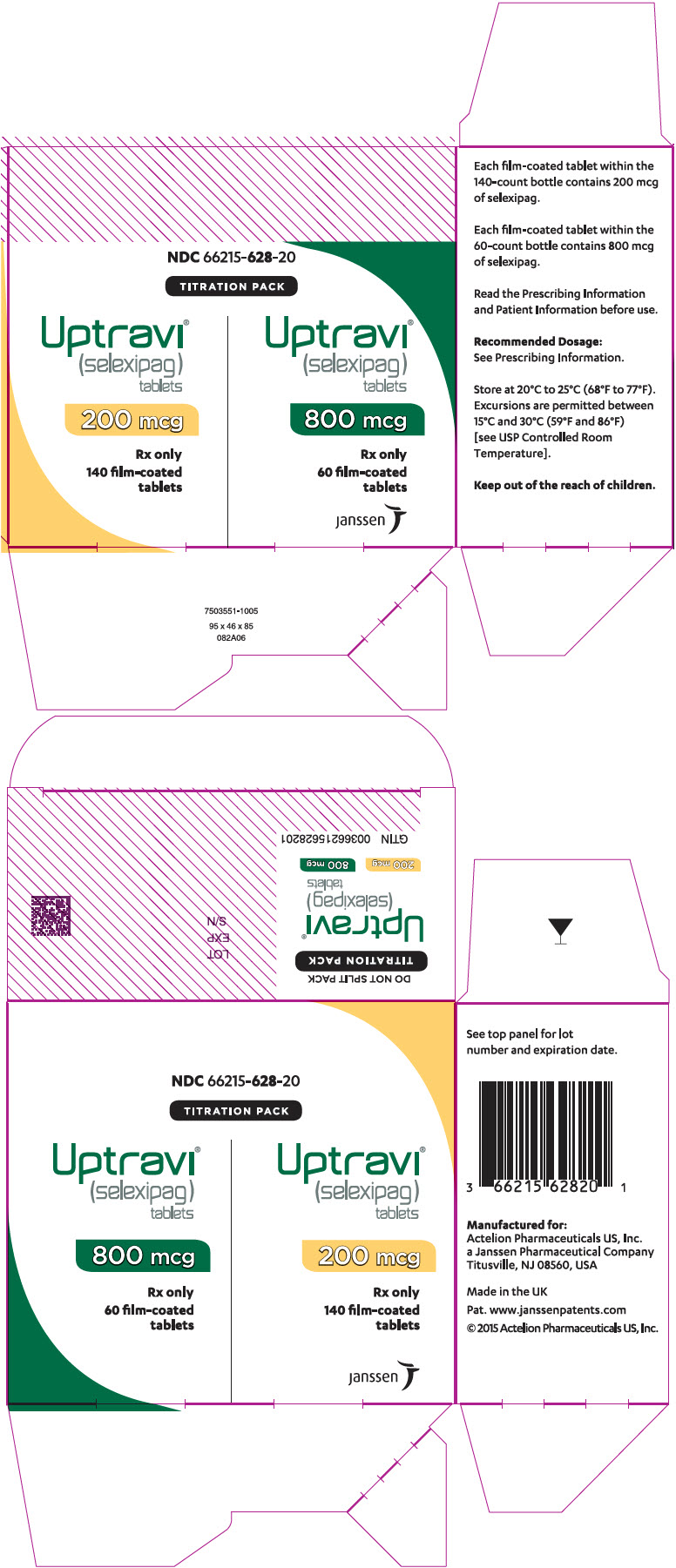
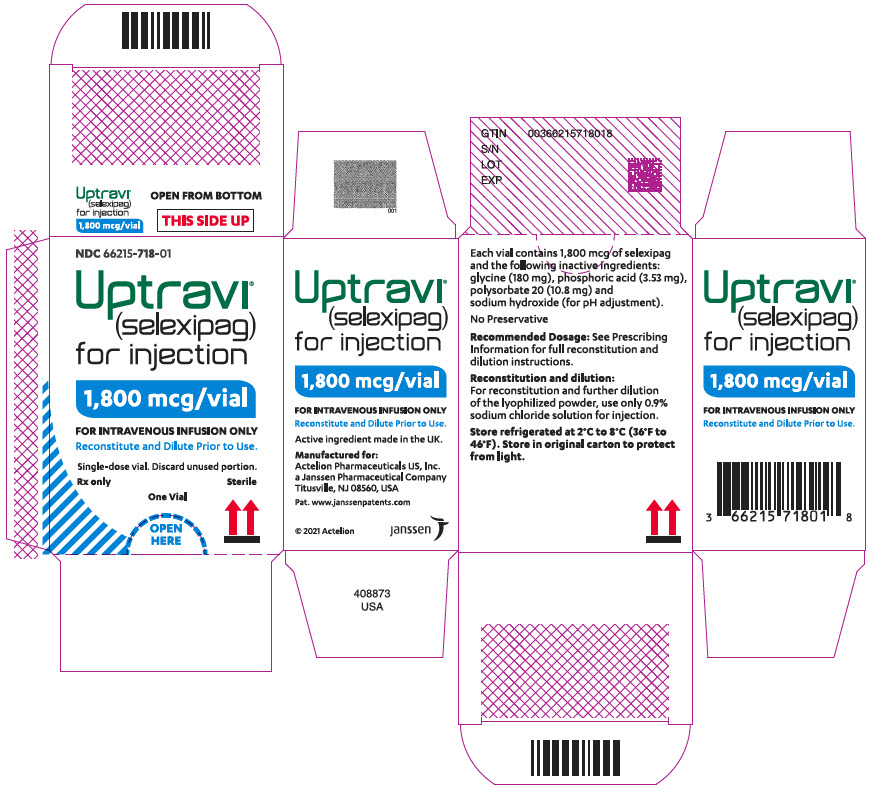
16PRINCIPAL DISPLAY PANEL - 1,800 mcg Vial Carton
NDC 66215-718-01
Uptravi
1,800 mcg/vial
FOR INTRAVENOUS INFUSION ONLY
Single-dose vial. Discard unused portion.
Rx only
One Vial
OPEN