Brand Name
Tavaborole
View Brand InformationFDA approval date: June 15, 2019
Classification: Oxaborole Antifungal
Form: Solution
What is Tavaborole?
Tavaborole topical solution is an oxaborole antifungal indicated for the treatment of onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes. Tavaborole topical solution is an oxaborole antifungal indicated for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Tavaborole (TAVABOROLE topical solution, 5%)
1INDICATIONS AND USAGE
Tavaborole topical solution, 5% is an oxaborole antifungal indicated for the treatment of onychomycosis of the toenails due to
2DOSAGE AND ADMINISTRATION
Apply tavaborole topical solution to affected toenails once daily for 48 weeks.
Tavaborole topical solution should be applied to the entire toenail surface and under the tip of each toenail being treated.
Tavaborole topical solution is for topical use only and not for oral, ophthalmic, or intravaginal use.
3DOSAGE FORMS AND STRENGTHS
Tavaborole topical solution, 5% is a clear, colorless alcohol-based solution. Each milliliter of solution contains 43.5 mg (5% w/w) of tavaborole
4CONTRAINDICATIONS
None.
5DESCRIPTION
Tavaborole topical solution, 5% contains tavaborole, 5% (w/w) in a clear, colorless alcohol-based solution for topical use. The active ingredient, tavaborole, is an oxaborole antifungal with the chemical name of 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole. The chemical formula is C
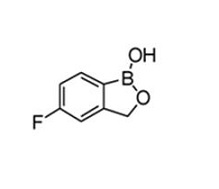
Tavaborole is a white to off-white powder. It is slightly soluble in water and freely soluble in ethanol and propylene glycol.
Each mL of tavaborole topical solution contains 43.5 mg of tavaborole. Inactive ingredients include alcohol USP (87.43% v/v), propylene glycol USP, and edetate calcium disodium USP.
6CLINICAL STUDIES
The efficacy and safety of tavaborole topical solution was evaluated in two multicenter, double-blind, randomized, vehicle-controlled trials. Tavaborole topical solution or vehicle was applied once daily for 48 weeks in subjects with 20% to 60% clinical involvement of the target toenail, without dermatophytomas or lunula (matrix) involvement.
A total of 1194 subjects (795 tavaborole topical solution, 399 Vehicle) 18 to 88 years of age, 82% male, 84% white, participated in these two trials. Efficacy assessments were made at 52 weeks following a 48-week treatment period.
The Complete Cure efficacy endpoint included negative mycology (negative KOH wet mount and negative fungal culture) and Completely Clear Nail (no clinical evidence of onychomycosis as evidenced by a normal toenail plate, no onycholysis, and no subungual hyperkeratosis). Efficacy results from the two trials are summarized in
* Complete cure defined as 0% clinical involvement of the target toenail plus negative KOH and negative culture.
† Complete or almost complete cure defined as ≤10% affected target toenail area involved and negative KOH and culture.
‡ Mycologic cure defined as negative KOH and negative culture.
† Complete or almost complete cure defined as ≤10% affected target toenail area involved and negative KOH and culture.
‡ Mycologic cure defined as negative KOH and negative culture.
7PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (
Manufactured by:
Distributed by:
8PATIENT INFORMATION
Tavaborole (ta va bor ole) topical solution, 5%
Important information: Tavaborole topical solution is for use on toenails only. Do not use tavaborole topical solution in your mouth, eyes, or vagina.
What is tavaborole topical solution?
Tavaborole topical solution is a prescription medicine used to treat fungal infections of the toenails.
It is not known if tavaborole topical solution is safe and effective in children less than 6 years age.
Before using tavaborole topical solution, tell your healthcare provider about all of your medical conditions, including if you:
• are pregnant or plan to become pregnant. It is not known if tavaborole topical solution can harm your unborn baby.
• are breastfeeding or plan to breastfeed. It is not known if tavaborole passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during your treatment with tavaborole topical solution.
• are pregnant or plan to become pregnant. It is not known if tavaborole topical solution can harm your unborn baby.
• are breastfeeding or plan to breastfeed. It is not known if tavaborole passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during your treatment with tavaborole topical solution.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use tavaborole topical solution?
See the “
• Use tavaborole topical solution exactly as your healthcare provider tells you to use it.
What should I avoid while using tavaborole topical solution?
• Tavaborole topical solution is flammable. Avoid heat and flame while applying tavaborole topical solution to your toenail.
• Tavaborole topical solution is flammable. Avoid heat and flame while applying tavaborole topical solution to your toenail.
What are the possible side effects of tavaborole topical solution?
The most common side effects of tavaborole topical solution include: skin peeling, ingrown toenail, redness, itching, and swelling.
Tavaborole topical solution may cause irritation at or near the application site. Tell your healthcare provider if you develop irritation at the application site that does not go away. These are not all of the possible side effects of tavaborole topical solution.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store tavaborole topical solution?
• Store tavaborole topical solution at room temperature, between 68°F to 77°F (20°C to 25°C).
Keep tavaborole topical solution and all medicines out of the reach of children.
General information about the safe and effective use of tavaborole topical solution
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use tavaborole topical solution for a condition for which it was not prescribed. Do not give tavaborole topical solution to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about tavaborole topical solution that is written for health professionals.
What are the ingredients in tavaborole topical solution?
Active ingredient: tavaborole
Inactive ingredients: alcohol USP (87.43% v/v), propylene glycol USP, and edetate calcium disodium USP
Manufactured by:
Distributed by:
For more information, call Encube Ethicals Private Limited at
This Patient Information has been approved by the U.S. Food and Drug Administration.
Rev.07/24
9Instructions for Use
Tavaborole (ta va bor ole) topical solution, 5%
Important information: Tavaborole topical solution is for use on toenails only. Do not use tavaborole topical solution in your mouth, eyes, or vagina.
Read the Instructions for Use that comes with tavaborole topical solution before you start using it. Talk to your healthcare provider if you have any questions.
How to apply tavaborole topical solution:
Your toenails should be clean and dry before you apply tavaborole topical solution.
Your toenails should be clean and dry before you apply tavaborole topical solution.
Step 1: Before you apply tavaborole topical solution to your affected toenail for the first time, remove the cap from the tavaborole topical solution bottle. (See Figure A) Throw away the cap.
Step 2: Remove the wrapping from the dropper that comes with tavaborole topical solution. insert the dropper into the tavaborole topical solution bottle. (See Figure B)
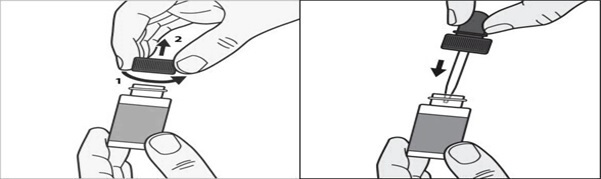
Figure AFigure B
Only apply tavaborole topical solution using the provided dropper. Do not use the dropper for any other purpose
Step 3: With the dropper inserted into the tavaborole topical solution, squeeze the bulb and then release the bulb to draw tavaborole topical solution into the dropper.
Step 4: Remove the dropper from the bottle and hold the dropper tip over your affected toenail. Slowly squeeze the bulb to apply tavaborole topical solution to your toenail. Apply enough solution to completely cover your toenail. You may need to use more than one drop. (See Figure C)
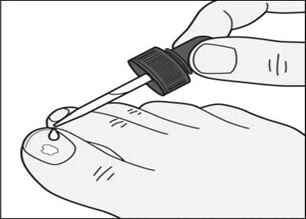
Figure C
Step 6: Use the dropper tip to gently spread tavaborole topical solution to cover the entire toenail up to the edges of the toenail. (See Figure D)
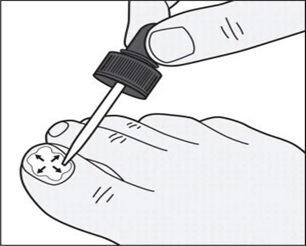
Figure D
Step 7: In addition to the top of the toenail, also apply tavaborole topical solution under the tip of the toenail. Use the dropper tip to gently spread tavaborole topical solution under the entire tip of the toenail. (See Figures E and F)
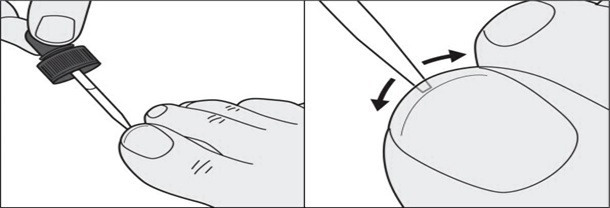
Figure EFigure F
Step 8: Repeat Steps 3 to 7 to apply tavaborole topical solution to each affected toenail.
Step 9: Let the tavaborole topical solution dry completely. This may take a couple of minutes. Avoid getting tavaborole topical solution on skin that is not surrounding skin, use a tissue to wipe any excess solution from the surrounding skin. Do not wipe tavaborole topical solution off of your toenails.
Step 10: After applying tavaborole topical solution to your toenails, insert the dropper back into the bottle and screw it on tightly.
Step 11: Wash your hands with soap and water after applying tavaborole topical solution.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Distributed by:
Rev.07/24
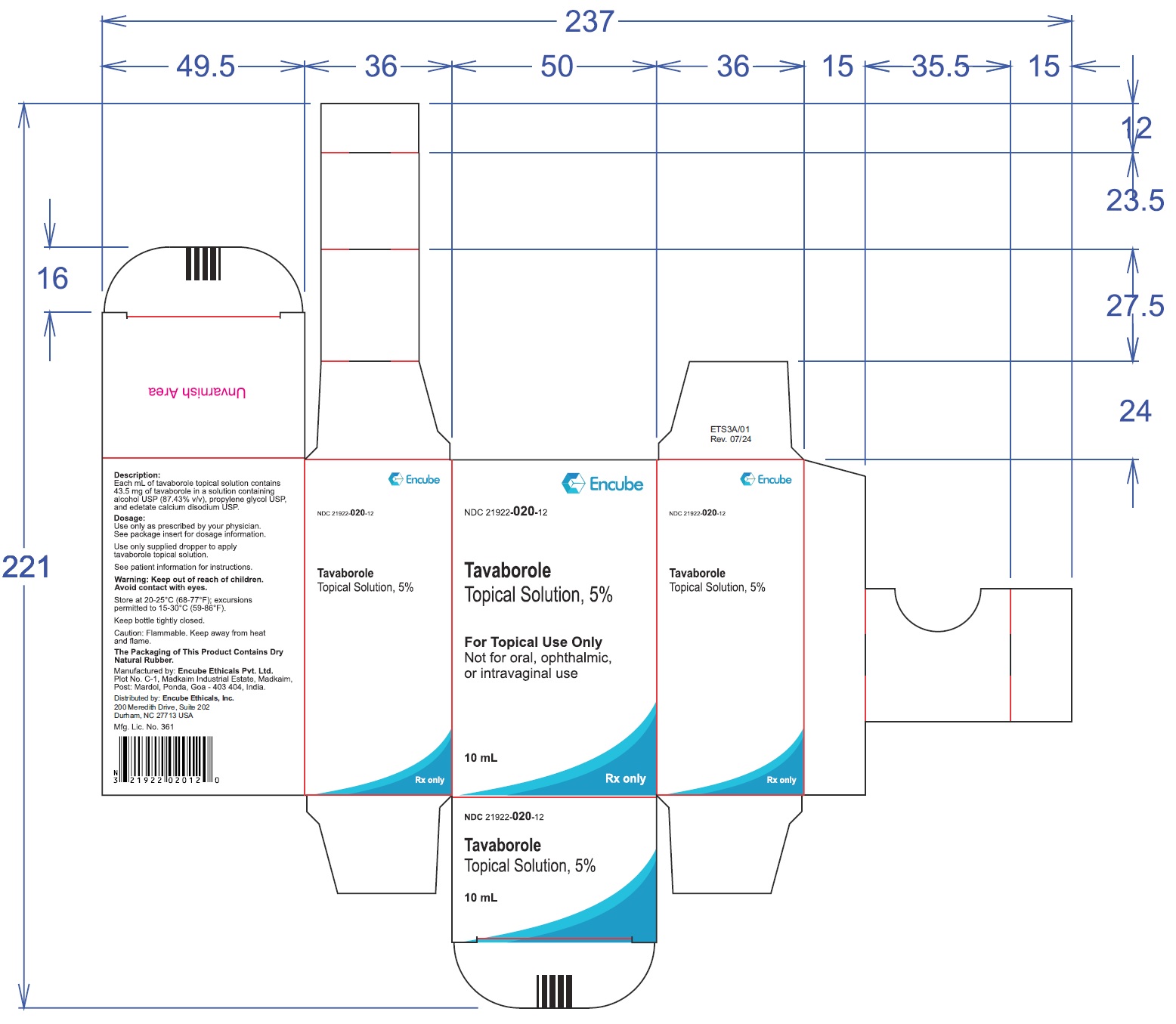
10PRINCIPAL DISPLAY PANEL
10 ml Carton Label:
NDC 21922-
Tavaborole Topical Solution, 5%
For Topical Use Only
Not for oral, ophthalmic, or intravaginal use
Not for oral, ophthalmic, or intravaginal use
10ml
Rx only