Generic Name
Pitavastatin
Brand Names
Zypitamag, Livalo
FDA approval date: February 03, 2017
Classification: HMG-CoA Reductase Inhibitor
Form: Tablet
What is Zypitamag (Pitavastatin)?
Pitavastatin tablets are indicated as an adjunct to diet to reduce low-density lipoprotein cholesterol in: Adults with primary hyperlipidemia. Adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia . Pitavastatin tablets are a HMG-CoA reductase inhibitor indicated as an adjunct to diet to reduce low-density lipoprotein cholesterol in: Adults with primary hyperlipidemia. Adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ZYPITAMAG (Pitavastatin Magnesium)
1INDICATIONS AND USAGE
ZYPITAMAG is indicated as an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia.
Pediatric use information is approved for Kowa Co Ltd LIVALO (pitavastatin) tablets. However, due to Kowa Co Ltd marketing exclusivity rights, this drug product is not labeled with that information.
2CONTRAINDICATIONS
ZYPITAMAG is contraindicated in the following conditions:
- Concomitant use of cyclosporine
- Acute liver failure or decompensated cirrhosis
- Hypersensitivity to pitavastatin or any excipients in ZYPITAMAG. Hypersensitivity reactions including angioedema, rash, pruritus, and urticaria have been reported with pitavastatin
3ADVERSE REACTIONS
The following serious adverse reactions are discussed in other sections of the labeling:
- Myopathy and Rhabdomyolysis
- Immune-Mediated Necrotizing Myopathy
- Hepatic Dysfunction
- Increases in HbA1c and Fasting Serum Glucose Levels
3.1Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of one drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults with Primary Hyperlipidemia
In 10 controlled clinical studies and 4 subsequent open-label extension studies, 3,291 adult patients with primary hyperlipidemia were administered pitavastatin 1 mg to 4 mg daily. The mean continuous exposure of pitavastatin (1 mg to 4 mg) was 36.7 weeks (median 51.1 weeks). The mean age of the patients was 60.9 years (range; 18 years – 89 years) and 52% females. Approximately 93% of the patients were White 7% were Asian/Indian, 0.2% were African American and 0.3% were Hispanic and other.
In 10 controlled clinical studies and 4 subsequent open-label extension studies, 3,291 adult patients with primary hyperlipidemia were administered pitavastatin 1 mg to 4 mg daily. The mean continuous exposure of pitavastatin (1 mg to 4 mg) was 36.7 weeks (median 51.1 weeks). The mean age of the patients was 60.9 years (range; 18 years – 89 years) and 52% females. Approximately 93% of the patients were White 7% were Asian/Indian, 0.2% were African American and 0.3% were Hispanic and other.
In controlled clinical studies and their open-label extensions, 3.9% (1 mg), 3.3% (2 mg), and 3.7% (4 mg) of pitavastatin-treated patients were discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: elevated creatine phosphokinase (0.6% on 4 mg) and myalgia (0.5% on 4 mg).
Adverse reactions reported in ≥ 2% of patients in controlled clinical studies and at a rate greater than or equal to placebo are shown in Table 1. These studies had treatment duration of up to 12 weeks.
Other adverse reactions reported from clinical studies were arthralgia, headache, influenza, and nasopharyngitis.
Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with pitavastatin.
The following laboratory abnormalities have been reported: elevated creatine phosphokinase, transaminases, alkaline phosphatase, bilirubin, and glucose.
Adverse Reactions in Adult HIV-Infected Patients with Dyslipidemia
In a double-blind, randomized, controlled, 52-week trial, 252 HIV-infected patients with dyslipidemia were treated with either pitavastatin 4 mg once daily (n=126) or another statin (n=126). All patients were taking antiretroviral therapy (excluding darunavir) and had HIV-1 RNA less than 200 copies/mL and CD4 count greater than 200 cell/μL for at least 3 months prior to randomization. The safety profile of pitavastatin was generally consistent with that observed in the clinical trials described above. One patient (0.8%) treated with pitavastatin had a peak creatine phosphokinase value exceeding 10 times the upper limit of normal (ULN), which resolved spontaneously. Four patients (3%) treated with pitavastatin had at least one ALT value exceeding 3 times but less than 5 times the ULN, none of which led to drug discontinuation. Virologic failure was reported for four patients (3%) treated with pitavastatin, defined as a confirmed measurement of HIV-1 RNA exceeding 200 copies/mL that was also more than a 2-fold increase from baseline.
Pediatric use information is approved for Kowa Co Ltd’s LIVALO (pitavastatin) tablets. However, due to Kowa Co Ltd’s marketing exclusivity rights, this drug product is not labeled with that information.
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pitavastatin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: abdominal discomfort, abdominal pain, dyspepsia, nausea
General disorders: asthenia, fatigue, malaise, dizziness
Hepatobiliary disorders: hepatitis, jaundice, fatal and non-fatal hepatic failure
Immune system disorders: angioedema, immune-mediated necrotizing myopathy associated with statin use
Metabolism and nutrition disorders: increases in HbA1c, fasting serum glucose levels
Musculoskeletal and connective tissue disorders: muscle spasms, myopathy, rhabdomyolysis
Nervous system disorders: hypoesthesia, peripheral neuropathy, rare reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. Cognitive impairment was generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). There have been rare reports of new-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when the same or a different statin was administered.
Psychiatric disorders: insomnia, depression.
Reproductive system and breast disorders: erectile dysfunction
Respiratory, thoracic and mediastinal disorders: interstitial lung disease
Skin and subcutaneous tissue disorders: lichen planus
4OVERDOSAGE
No specific treatment for pitavastatin overdose is known. Contact Poison Control (1-800-222-1222) for latest recommendations. Hemodialysis is unlikely to be of benefit due to high protein binding ratio of pitavastatin.
5DESCRIPTION
ZYPITAMAG (pitavastatin) tablets for oral use is an HMG-CoA reductase inhibitor.
The chemical name for pitavastatin is (3R,5S)-7-[2-Cyclopropyl-4-(4-fluorophenyl) quinoline-3-yl]3,5-dihydroxy-6(E)-heptanoic acid hemi magnesium. The structural formula is:
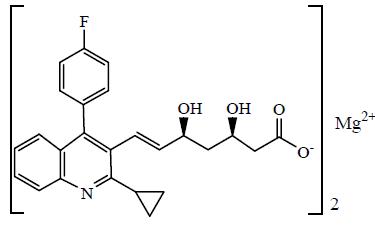
The molecular formula for pitavastatin is C
Each film-coated tablet of ZYPITAMAG contains 2.053 mg or 4.106 mg of pitavastatin magnesium, which is equivalent to 2 mg or 4 mg, respectively of free base and the following inactive ingredients: calcium carbonate, crospovidone, hypromellose, lactose monohydrate, magnesium stearate and sodium carbonate anhydrous and film-coating containing the following inactive ingredients: hypromellose, polyethylene glycol, talc and titanium dioxide.
6HOW SUPPLIED/STORAGE AND HANDLING
ZYPITAMAG (pitavastatin) Tablets, 2 mg are white to off-white, beveled-edge, round-shaped tablets debossed with “877” on one side and plain on the other side and are supplied as follows:
NDC 25208-201-09 in bottle of 90 tablets with child-resistant closure
ZYPITAMAG (pitavastatin) Tablets, 4 mg are white to off-white, beveled-edge, round-shaped tablets
NDC 25208-202-09 in bottle of 90 tablets with child-resistant closure
Storage
Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Protect from moisture and light.
Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Protect from moisture and light.
7PATIENT COUNSELING INFORMATION
Myopathy and Rhabdomyolysis
Advise patients that ZYPITAMAG may cause myopathy and rhabdomyolysis. Inform patients that the risk is increased when taking certain types of medication and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever
Hepatic Dysfunction
Inform patients that ZYPITAMAG may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (.
Inform patients that ZYPITAMAG may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (.
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with ZYPITAMAG. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (.
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with ZYPITAMAG. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (.
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if ZYPITAMAG should be discontinued [see Use in Specific Populations (
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if ZYPITAMAG should be discontinued [see Use in Specific Populations (
Lactation
Advise patients that breastfeeding is not recommended during treatment with ZYPITAMAG [see Use in Specific Populations (.
Advise patients that breastfeeding is not recommended during treatment with ZYPITAMAG [see Use in Specific Populations (.
Please address medical inquiries to (medical.information@medicure.com) Tel.: 1-800-509-0544.
This product’s label may have been updated. For current full prescribing information, please visit www.medicure.com
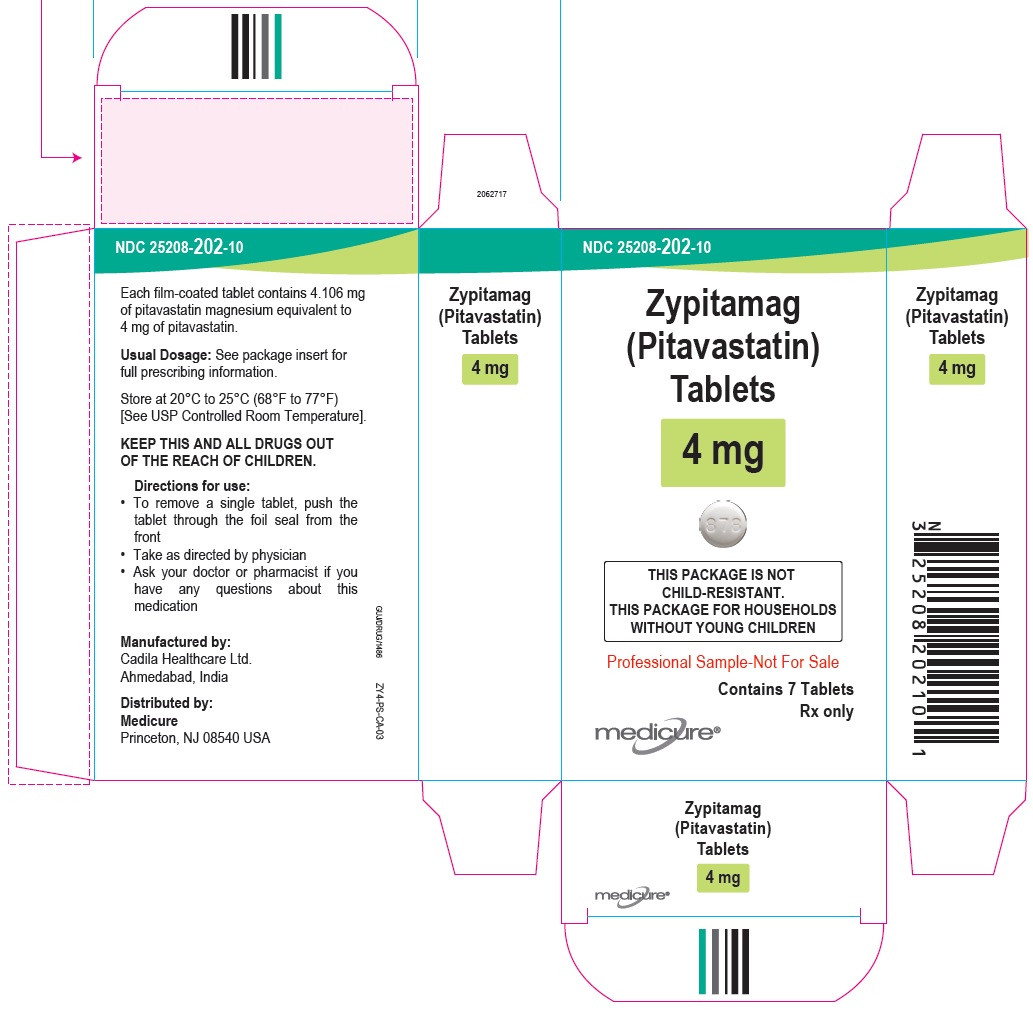
8PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
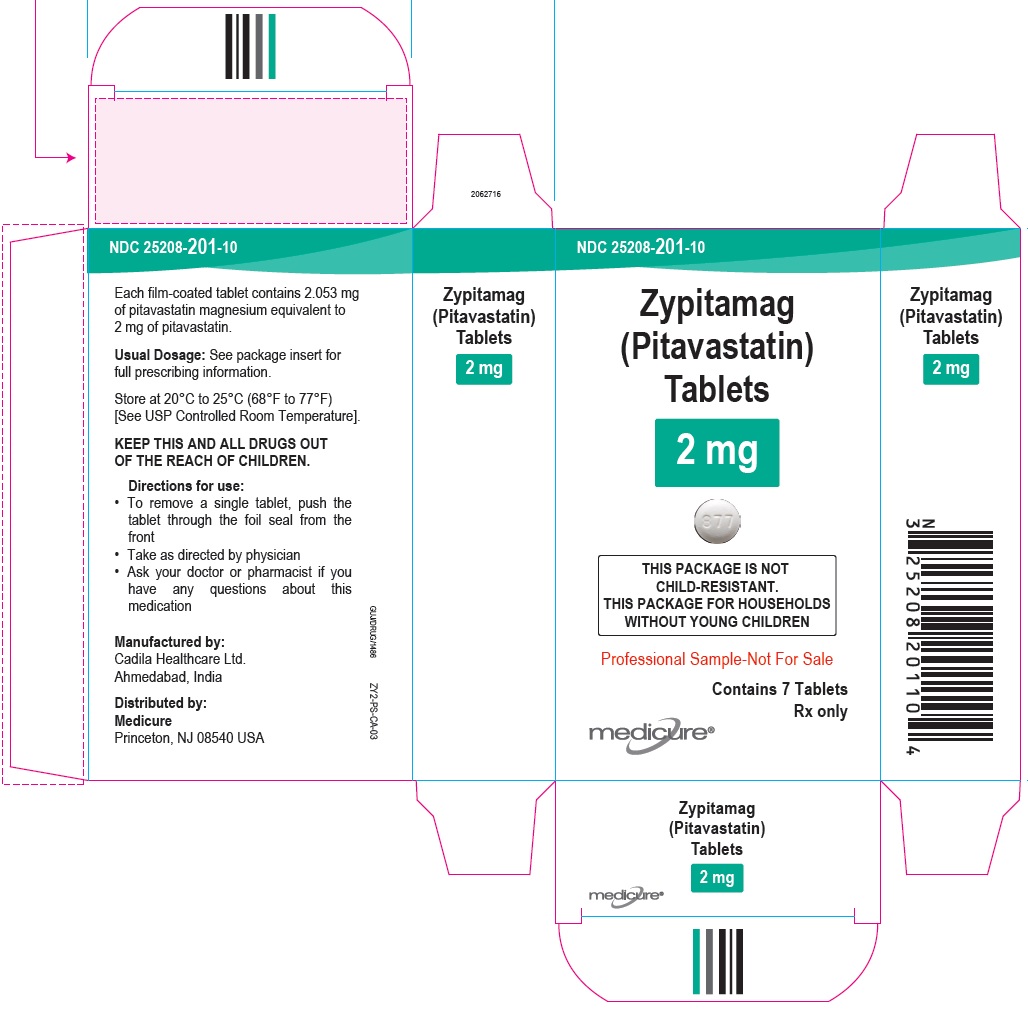
NDC 25208-201-09
Zypitamag (Pitavastatin) Tablets, 2 mg
90 Tablets
Rx only

NDC 25208-201-15
Zypitamag (Pitavastatin) Tablets, 2 mg
500 Tablets in Bottle
Rx only
NDC 25208-202-09
Zypitamag (Pitavastatin) Tablets, 4 mg
90 Tablets
Rx only

NDC 25208-202-15
Zypitamag (Pitavastatin) Tablets, 4 mg
500 Tablets in Bottle
Rx only