Brand Name
Northera
Generic Name
Droxidopa
View Brand Information FDA approval date: September 01, 2014
Form: Capsule
What is Northera (Droxidopa)?
Droxidopa capsules are indicated for the treatment of orthostatic dizziness, lightheadedness, or the “feeling that you are about to black out” in adult patients with symptomatic neurogenic orthostatic hypotension caused by primary autonomic failure (Parkinson's disease , multiple system atrophy, and pure autonomic failure), dopamine beta-hydroxylase deficiency, and non-diabetic autonomic neuropathy. Effectiveness beyond 2 weeks of treatment has not been established. The continued effectiveness of droxidopa capsules should be assessed periodically. Droxidopa capsules are indicated for the treatment of orthostatic dizziness, lightheadedness, or the “feeling that you are about to black out” in adult patients with symptomatic neurogenic orthostatic hypotension caused by primary autonomic failure (Parkinson's disease , multiple system atrophy, and pure autonomic failure), dopamine beta-hydroxylase deficiency, and non-diabetic autonomic neuropathy. Effectiveness beyond 2 weeks of treatment has not been established. The continued effectiveness of droxidopa capsules should be assessed periodically .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Northera (droxidopa)
WARNING: SUPINE HYPERTENSION
Monitor supine blood pressure prior to and during treatment and more frequently when increasing doses. Elevating the head of the bed lessens the risk of supine hypertension, and blood pressure should be measured in this position. If supine hypertension cannot be managed by elevation of the head of the bed, reduce or discontinue NORTHERA[see Warnings and Precautions (
1INDICATIONS AND USAGE
NORTHERA is indicated for the treatment of orthostatic dizziness, lightheadedness, or the “feeling that you are about to black out” in adult patients with symptomatic neurogenic orthostatic hypotension (nOH) caused by primary autonomic failure (Parkinson's disease [PD], multiple system atrophy, and pure autonomic failure), dopamine beta-hydroxylase deficiency, and non-diabetic autonomic neuropathy. Effectiveness beyond 2 weeks of treatment has not been established. The continued effectiveness of NORTHERA should be assessed periodically.
2DOSAGE FORMS AND STRENGTHS
NORTHERA capsules are available in 100 mg, 200 mg, and 300 mg strengths as specified below.
- 100 mg: Hard gelatin capsules with “Northera” on the white body and “100” on the light blue cap
- 200 mg: Hard gelatin capsules with “Northera” on the white body and “200” on the light yellow cap
- 300 mg: Hard gelatin capsules with “Northera” on the white body and “300” on the light green cap
3CONTRAINDICATIONS
NORTHERA is contraindicated in patients who have a history of hypersensitivity to the drug or its ingredients
4ADVERSE REACTIONS
The following adverse reactions with NORTHERA are included in more detail in the Warnings and Precautions section of the label:
- Supine Hypertension
- Hyperpyrexia and Confusion
- May exacerbate existing ischemic heart disease, arrhythmias, and congestive heart failure
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety evaluation of NORTHERA is based on two placebo-controlled studies 1 to 2 weeks in duration (Studies 301 and 302), one 8-week placebo-controlled study (Study 306), and two long-term, open-label extension studies (Studies 303 and 304). In the placebo-controlled studies, a total of 485 patients with Parkinson's disease, multiple system atrophy, pure autonomic failure, dopamine beta-hydroxylase deficiency, or non-diabetic autonomic neuropathy were randomized and treated, 245 with NORTHERA and 240 with placebo
Placebo-Controlled Experience
The most commonly observed adverse reactions (those occurring at an incidence of greater than 5% in the NORTHERA group and with at least a 3% greater incidence in the NORTHERA group than in the placebo group) in NORTHERA-treated patients during the three placebo-controlled trials were headache, dizziness, nausea, and hypertension. The most common adverse reactions leading to discontinuation from NORTHERA were hypertension or increased blood pressure and nausea.
Note: n=number of patients. Adverse reactions that were reported in greater than 5% of patients in the NORTHERA group and with at least a 3% greater incidence in the NORTHERA group than in the placebo group were from Study 306.
Long-Term, Open-Label Trials with NORTHERA
In the long-term, open-label extension studies, a total of 422 patients, mean age 65 years, were treated with NORTHERA for a mean total exposure of approximately one year. The commonly reported adverse events were falls (24%), urinary tract infections (15%), headache (13%), syncope (13%), and dizziness (10%).
In the long-term, open-label extension studies, a total of 422 patients, mean age 65 years, were treated with NORTHERA for a mean total exposure of approximately one year. The commonly reported adverse events were falls (24%), urinary tract infections (15%), headache (13%), syncope (13%), and dizziness (10%).
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of NORTHERA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: Chest pain
Eye Disorders: Blurred vision
Gastrointestinal Disorders: Pancreatitis, abdominal pain, vomiting, diarrhea
General Disorders and Administration Site Conditions: Fatigue
Nervous System Disorders: Cerebrovascular accident
Psychiatric Disorders: Psychosis,hallucination, delirium, agitation, memory disorder
Eye Disorders: Blurred vision
Gastrointestinal Disorders: Pancreatitis, abdominal pain, vomiting, diarrhea
General Disorders and Administration Site Conditions: Fatigue
Nervous System Disorders: Cerebrovascular accident
Psychiatric Disorders: Psychosis,hallucination, delirium, agitation, memory disorder
5DESCRIPTION
NORTHERA capsules contain droxidopa, which is a synthetic amino acid precursor of norepinephrine, for oral administration. Chemically, droxidopa is (–)-threo-3-(3,4-Dihydroxyphenyl)-L-serine. It has the following structural formula:
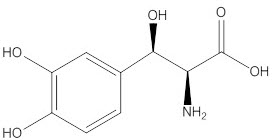
Droxidopa is an odorless, tasteless, white to off-white crystals or crystalline powder. It is slightly soluble in water, and practically insoluble in methanol, glacial acetic acid, ethanol, acetone, ether, and chloroform. It is soluble in dilute hydrochloric acid. It has a molecular weight of 213.19 and a molecular formula of C
NORTHERA capsules also contain the following inactive ingredients: mannitol, corn starch, and magnesium stearate. The capsule shell is printed with black ink. The black inks contain shellac glaze, ethanol, iron oxide black, isopropyl alcohol, n-butyl alcohol, propylene glycol, and ammonium hydroxide. The capsule shell contains the following inactive ingredients: 100 mg – gelatin, titanium dioxide, FD&C Blue No. 2, black and red iron oxide; 200 mg – gelatin, titanium dioxide, FD&C Blue No. 2, black and yellow iron oxide; 300 mg – gelatin, titanium dioxide, FD&C Blue No. 1, FD&C Yellow No. 5 (tartrazine), and FD&C Red No. 40. NORTHERA capsules differ in size and color by strength
6PATIENT COUNSELING INFORMATION
Elevations in Blood PressureCounsel patients that NORTHERA causes elevations in blood pressure and increases the risk of supine hypertension, which could lead to strokes, heart attacks, and death. Instruct patients to rest and sleep in an upper-body elevated position and monitor blood pressure. Instruct patients how to manage observed blood pressure elevations. To reduce the risk of supine hypertension, in addition to raising the upper body, the late afternoon dose of NORTHERA should be taken at least three hours before bedtime [see Warnings and Precautions (.
Concomitant TreatmentsCounsel patients about the concomitant use of drugs to treat other conditions that may have an additive effect with NORTHERA [see Drug Interactions (
Allergic ReactionsCounsel patients to discontinue NORTHERA and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction such as anaphylaxis, angioedema, bronchospasm, urticaria or rash occur [see Warnings and Precautions (
Lactation
Advise women not to breastfeed during treatment with NORTHERA
FoodPatients should take NORTHERA the same way each time, either with food or without food [see Dosage and Administration (.
Missed DoseIf a dose is missed, patients should take the next dose at the regularly scheduled time and should not double the dose.
Manufactured by:
For:
NORTHERA is a registered trademark of Lundbeck NA Ltd.
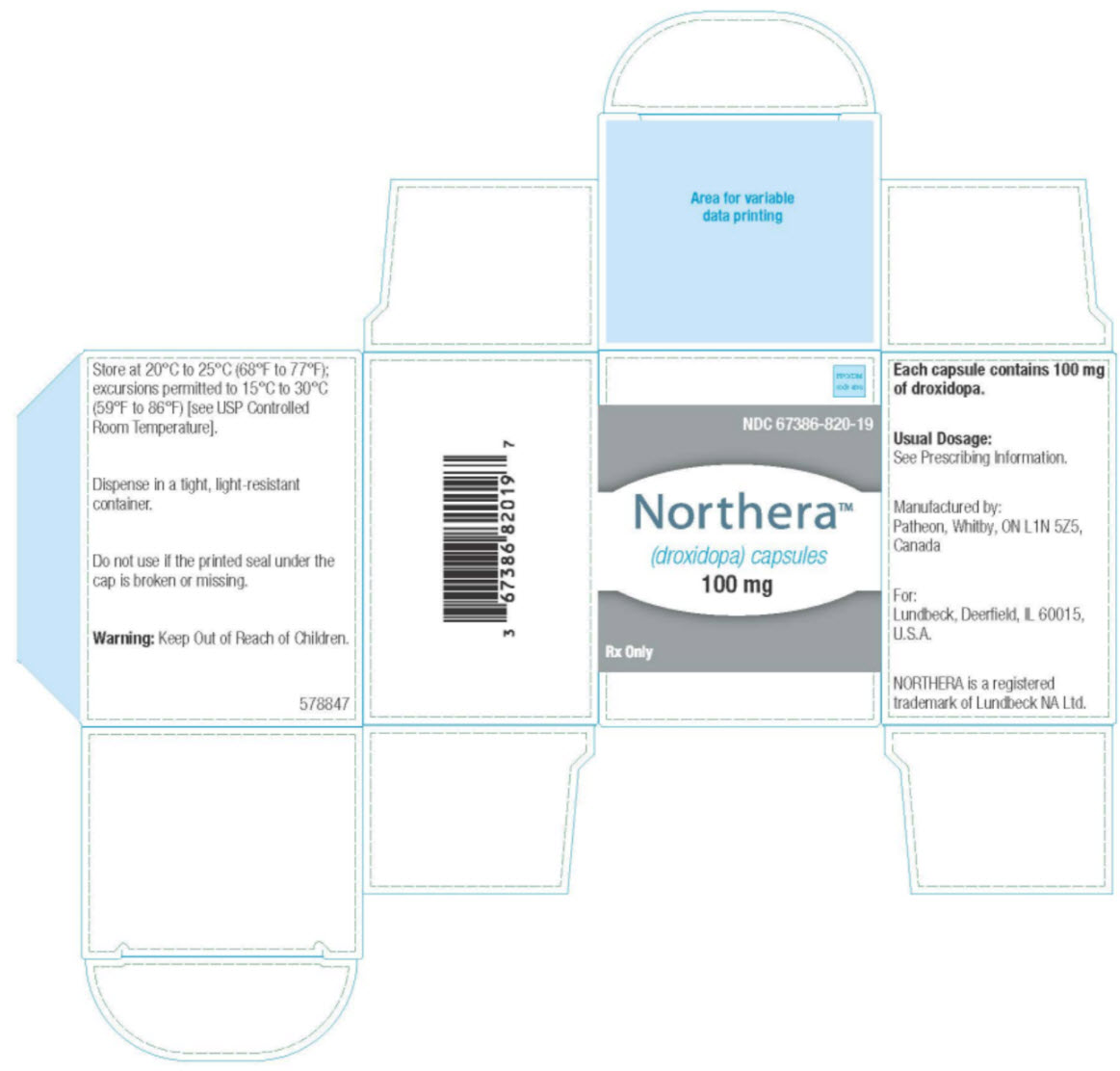
7PRINCIPAL DISPLAY PANEL
NDC 67386-820-19
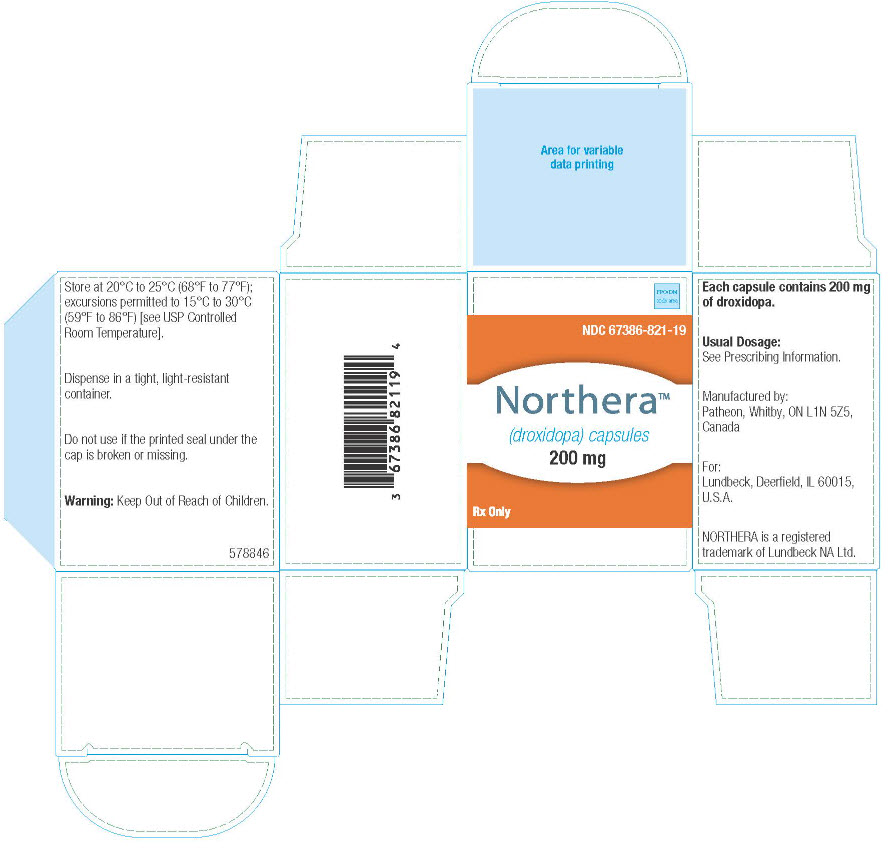
8PRINCIPAL DISPLAY PANEL
NDC 67386-821-19
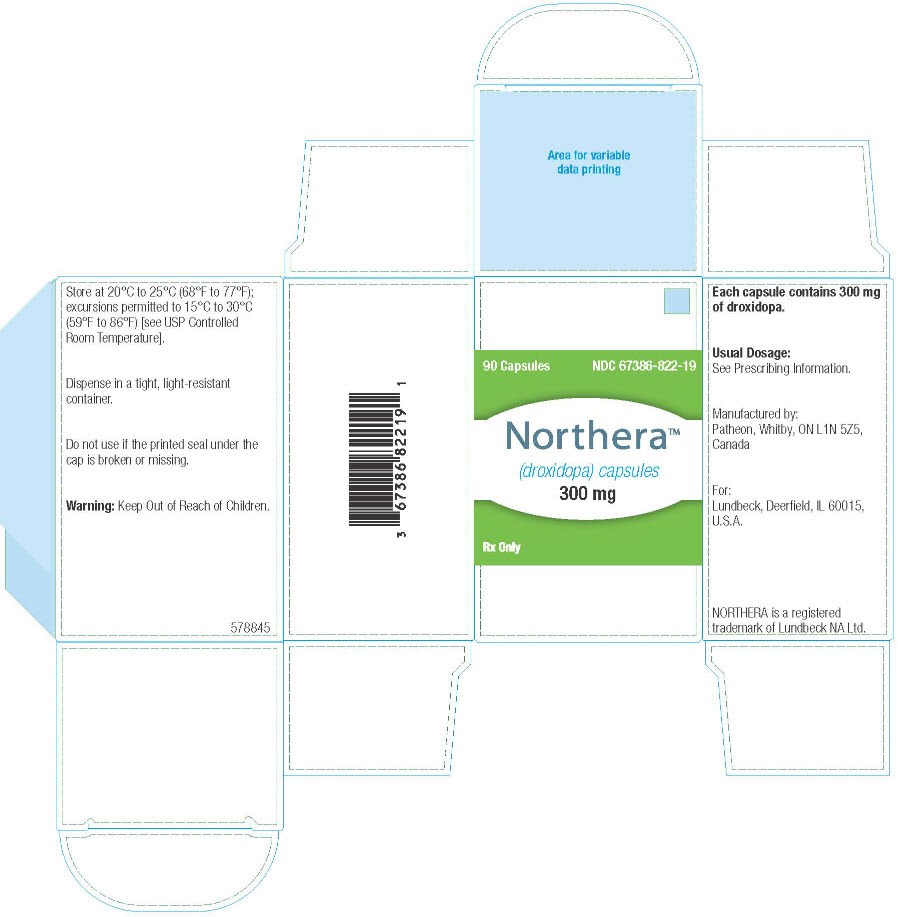
9PRINCIPAL DISPLAY PANEL
NDC 67386-822-19
10PRINCIPAL DISPLAY PANEL
NDC 67386-820-99
Professional Sample
