Generic Name
Lactulose
Brand Names
Constulose, Enulose, Generlac, Kristalose
FDA approval date: July 30, 1966
Classification: Osmotic Laxative
Form: Powder, Solution
What is Constulose (Lactulose)?
For the prevention and treatment of portal-systemic encephalopathy, including the stages of hepatic pre-coma and coma. Controlled studies have shown that lactulose solution therapy reduces the blood ammonia level by 25 to 50%; this is generally paralleled by an improvement in the patients’ mental state and by an improvement in EEG patterns. The clinical response has been observed in about 75% of patients, which is at least as satisfactory as that resulting from neomycin therapy. An increase in patients’ protein tolerance is also frequently observed with lactulose solution therapy. In the treatment of chronic portal-systemic encephalopathy, lactulose solution has been given for over 2 years in controlled studies.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Constulose (Lactulose)
1DESCRIPTION
Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution, USP contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 0.1 g or less of fructose).
Lactulose is a colonic acidifier which promotes laxation.
The chemical name for lactulose is 4-O-
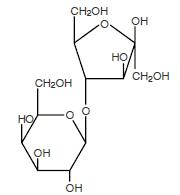
The molecular weight is 342
2CLINICAL PHARMACOLOGY
Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool.
Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement.
Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours.
3INDICATIONS AND USAGE
For the treatment of constipation. In patients with a history of chronic constipation, lactulose solution therapy increases the number of bowel movements per day and the number of days on which bowel movements occur.
4CONTRAINDICATIONS
Since lactulose solution contains galactose (less than 1.6 g/15 mL), it is contraindicated in patients who require a low galactose diet.
5WARNINGS
A theoretical hazard may exist for patients being treated with lactulose solution who may be required to undergo electrocautery procedures during proctoscopy or colonoscopy. Accumulation of H
6ADVERSE REACTIONS
Precise frequency data are not available.
Initial dosing may produce flatulence and intestinal cramps, which are usually transient. Excessive dosage can lead to diarrhea with potential complications such as loss of fluids, hypokalemia, and hypernatremia.
Nausea and vomiting have been reported.
To report SUSPECTED ADVERSE EVENTS, contact the FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
7OVERDOSAGE
Signs and Symptoms:
There have been no reports of accidental overdosage. In the event of overdosage, it is expected that diarrhea and abdominal cramps would be the major symptoms. Medication should be terminated.
Oral LD50: The acute oral LD50 of the drug is 48.8 mL/kg in mice and greater than 30 mL/kg in rats.
Dialysis: Dialysis data are not available for lactulose. Its molecular similarity to sucrose, however, would suggest that it should be dialyzable.
8DOSAGE AND ADMINISTRATION
The usual dose is 1 to 2 tablespoonfuls (15 to 30 mL, containing 10 g to 20 g of lactulose) daily. The dose may be increased to 60 mL daily if necessary. Twenty-four to 48 hours may be required to produce a normal bowel movement.
Note: Some patients have found that lactulose solution may be more acceptable when mixed with fruit juice, water or milk.
9HOW SUPPLIED
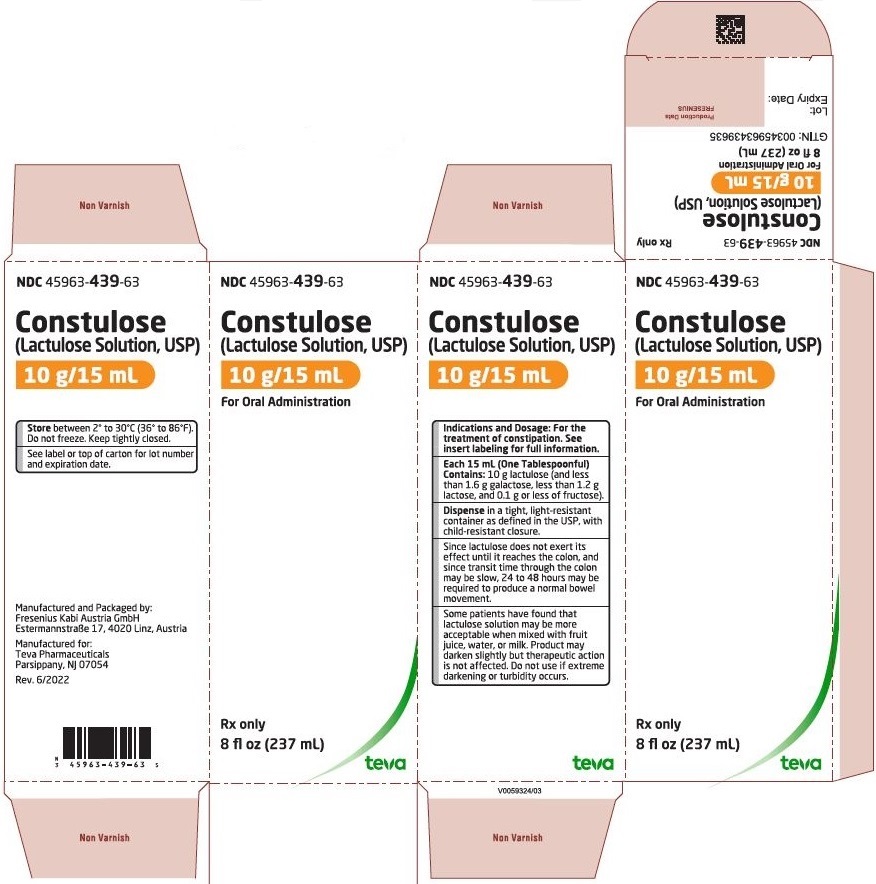
Lactulose Solution, USP is a natural colored and an unflavored solution available in 8 fl oz (237 mL) and 1 quart (946 mL) bottles.
Lactulose Solution, USP contains lactulose 670 mg/mL (10 g/15 mL).
Store between 36° to 86°F (2° to 30°C). Do not freeze.
Under recommended storage conditions, a normal darkening of color may occur. Such darkening is characteristic of sugar solutions and does not affect therapeutic action. Prolonged exposure to temperatures above 86°F (30°C) or to direct light may cause extreme darkening and turbidity which may be pharmaceutically objectionable. If this condition develops, do not use.
Prolonged exposure to freezing temperatures may cause change to a semi-solid, too viscous to pour. Viscosity will return to normal upon warming to room temperature.
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure.
Manufactured and packaged by:
Manufactured for:
Revised – June 2022
10PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 45963-439-63
Constulose
Rx only