Brand Name
Intelence
Generic Name
Etravirine
View Brand Information FDA approval date: January 18, 2008
Classification: Human Immunodeficiency Virus 1 Non-Nucleoside Analog Reverse Transcriptase Inhibitor
Form: Tablet
What is Intelence (Etravirine)?
Etravirine, in combination with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus type 1 infection in antiretroviral treatment-experienced adult patients and pediatric patients 2 years of age and older [see Microbiology (1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Intelence (etravirine)
1INDICATIONS AND USAGE
INTELENCE, in combination with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced adult patients and pediatric patients 2 years of age and older
2DOSAGE FORMS AND STRENGTHS
- 25 mg white to off-white, oval, scored tablets debossed with "TMC" on one side.
- 100 mg white to off-white oval tablets debossed with "TMC125" on one side and "100" on the other side.
- 200 mg white to off-white, biconvex, oblong tablets debossed with "T200" on one side.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Severe skin and hypersensitivity reactions
- Immune reconstitution syndrome
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following events have been identified during postmarketing use of INTELENCE. Because these events are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Severe hypersensitivity reactions including DRESS and cases of hepatic failure have been reported [see .
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis
Skin and Subcutaneous Tissue Disorders: Fatal cases of toxic epidermal necrolysis and Stevens-Johnson syndrome have been reported [see .
5OVERDOSAGE
There is no specific antidote for overdose with INTELENCE. Human experience of overdose with INTELENCE is limited. The highest dose studied in healthy volunteers was 400 mg once daily. Treatment of overdose with INTELENCE consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. Because etravirine is highly protein bound, dialysis is unlikely to result in significant removal of the active substance.
6DESCRIPTION
INTELENCE
The chemical name for etravirine is 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile. Its molecular formula is C
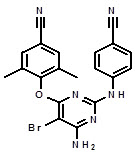
Etravirine is a white to slightly yellowish-brown powder. Etravirine is practically insoluble in water over a wide pH range. It is very slightly soluble in propylene glycol and slightly soluble in ethanol. Etravirine is soluble in polyethylene glycol (PEG)400 and freely soluble in some organic solvents (e.g., N,N-dimethylformamide and tetrahydrofuran).
INTELENCE
INTELENCE
INTELENCE
7HOW SUPPLIED/STORAGE AND HANDLING
INTELENCE
INTELENCE
INTELENCE
INTELENCE tablets are packaged in bottles in the following configuration:
- 25 mg tablets—bottles of 120 (NDC 59676-572-01). Each bottle contains 2 desiccant pouches.
- 100 mg tablets—bottles of 120 (NDC 59676-570-01). Each bottle contains 3 desiccant pouches.
- 200 mg tablets—bottles of 60 (NDC 59676-571-01). Each bottle contains 3 desiccant pouches.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
9PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
120 Tablets
INTELENCE
100 mg
Each tablet contains
Rx only
janssen
ALERT: Find out about medicines that

10PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
60 Tablets
INTELENCE
200 mg
Each tablet contains
Rx only
janssen
ALERT: Find out about medicines that

11PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label
120 Tablets
INTELENCE
25 mg
Each tablet contains 25 mg of etravirine.
Rx only
ALERT: Find out about medicines that
