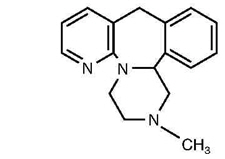
Mirtazapine
What is Remeron (Mirtazapine)?
Approved To Treat
Related Clinical Trials
Summary: This study is a single-center, parallel and double-blind study in Movement Disorders Clinic of Shohadaye Tajrish Hospital. Patients, researchers (physicians, outcome assessors) and data analysts are blinded. After assessing the inclusion and exclusion criteria's, patients who assigned the informed consent form, are randomly divided into control and treatment groups.Patients in treatment or placebo...
Summary: Functional Dyspepsia (FD) is diagnosed in the presence of bothersome epigastric pain or burning, early satiation and/or postprandial fullness of greater than 8 weeks duration, in the absence of alarm signs. Alarm signs include weight loss, gastrointestinal bleeding, anemia, dysphagia, and family history of upper gastrointestinal malignancies. FD is a common gastrointestinal complaint. It's prevale...
Summary: This project will evaluate the ability of Mirtazapine (MZP), a pharmacologically unique medication with a growing body of evidence to support its efficacy and safety for the treatment of methamphetamine (MA) use among medication for opioid use disorder (MOUD) patients, to significantly decrease MA use and related health-impairing behaviors. MZP has already successfully been used in the treatment o...
Related Latest Advances
Brand Information
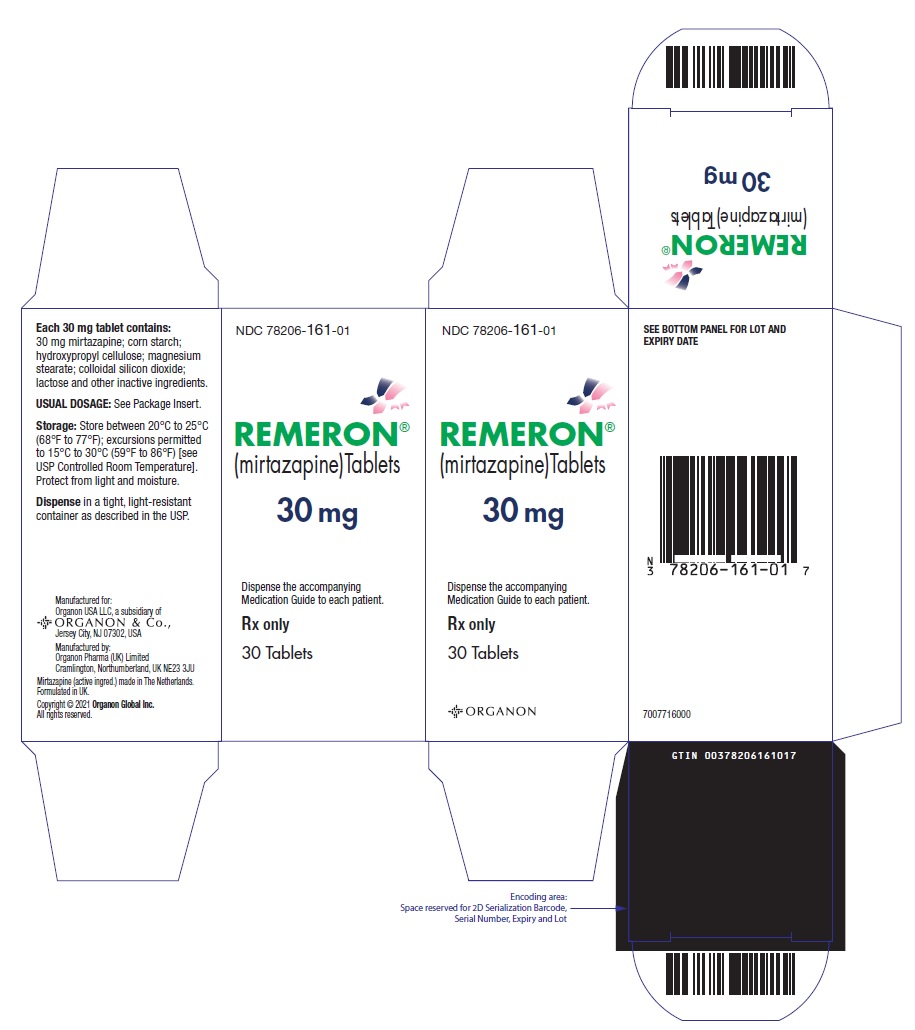
- 15 mg tablets: Oval, scored, yellow, with "MSD" debossed on one side and "
- 30 mg tablets: Oval, scored, red-brown, with "MSD" debossed on one side and "
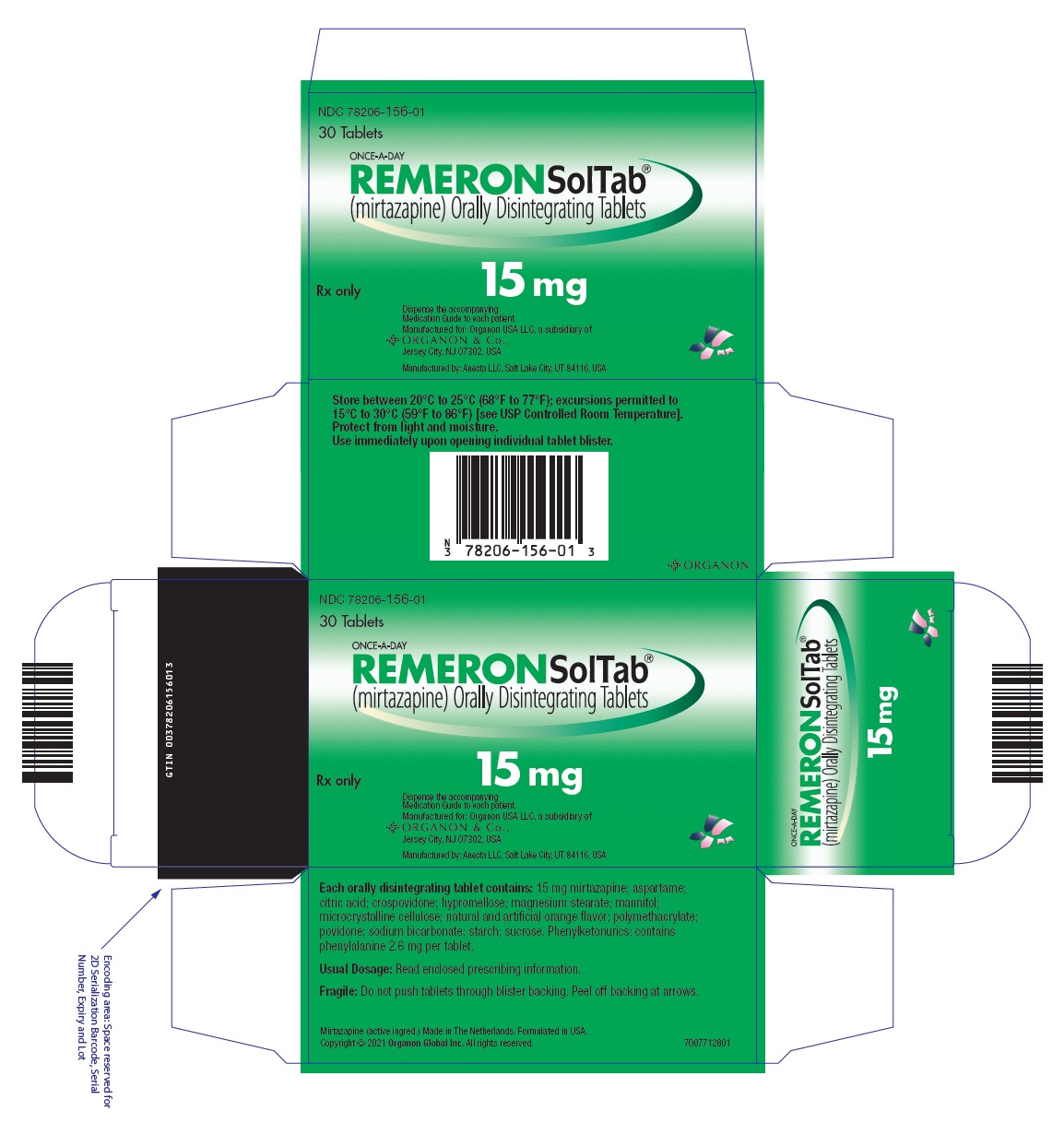
- 15 mg orally disintegrating tablets: Round, white, with "
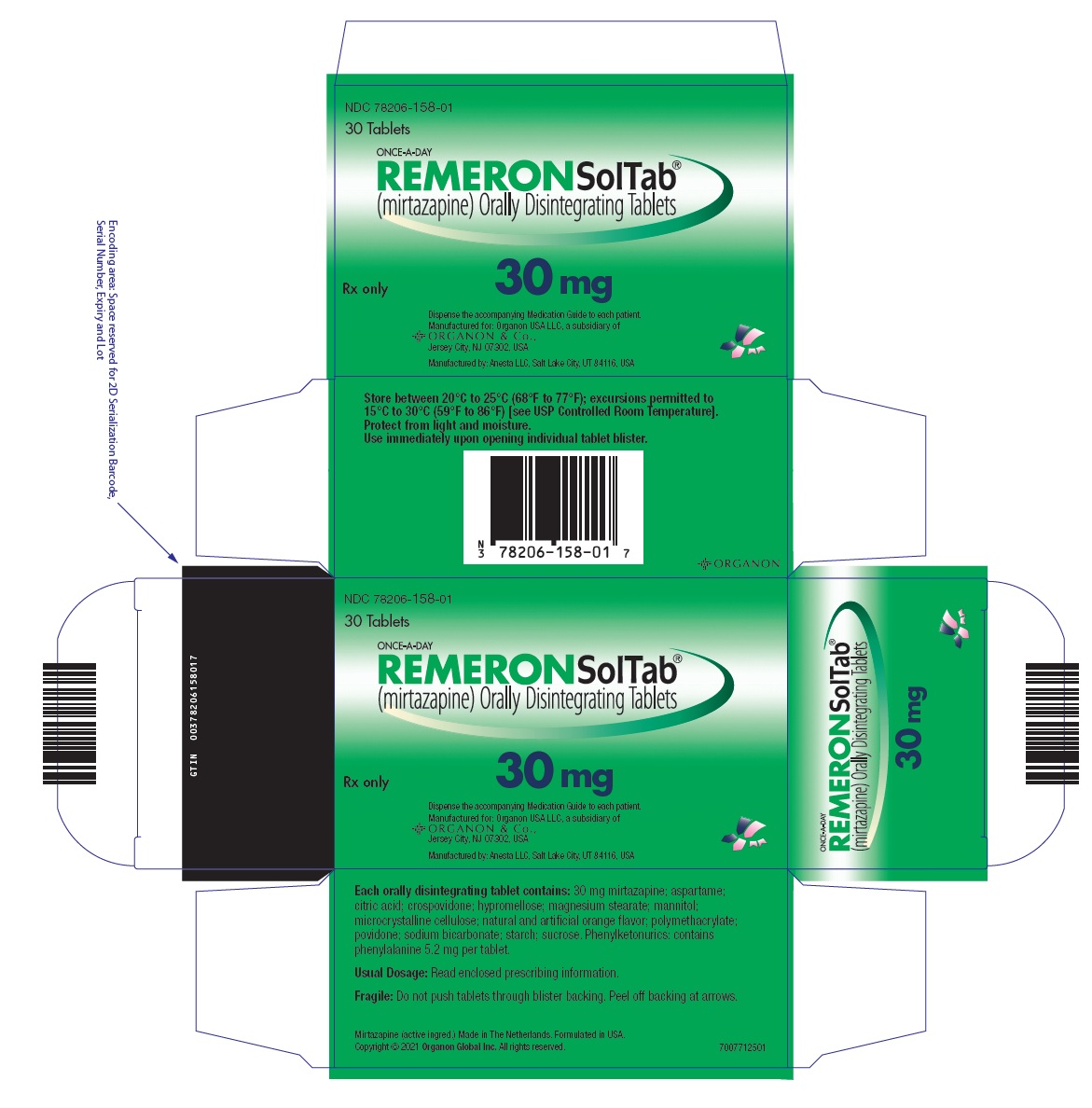
- 30 mg orally disintegrating tablets: Round, white, with "
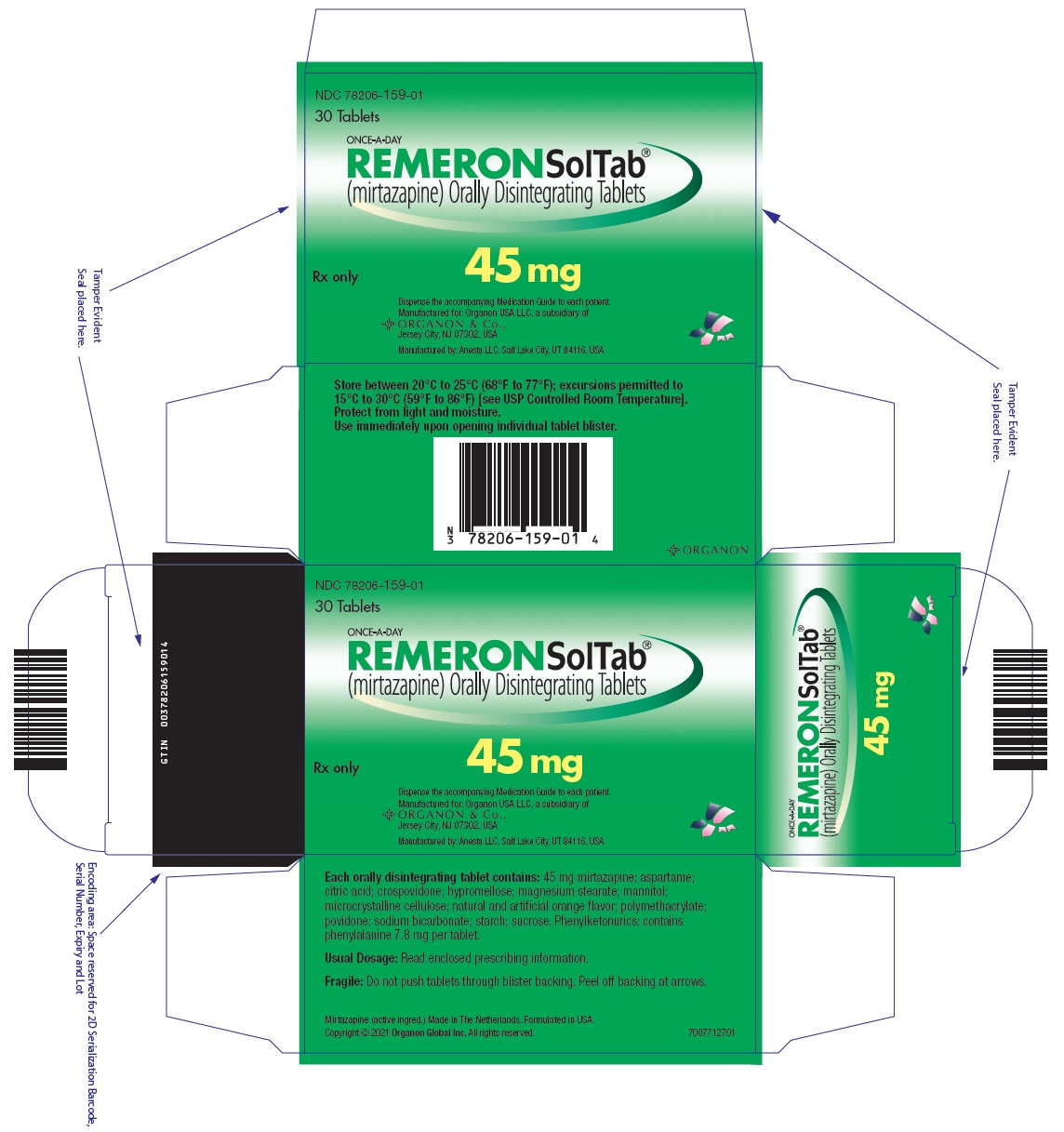
- 45 mg orally disintegrating tablets: Round, white, with "
- Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of an increased risk of serotonin syndrome
- With a known hypersensitivity to mirtazapine or to any of the excipients in REMERON/REMERONSolTab
- Hypersensitivity
- Suicidal Thoughts and Behaviors
- Agranulocytosis
- Serotonin Syndrome
- Angle-Closure Glaucoma
- QT Prolongation and Torsades de Pointes
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
- Increased Appetite and Weight Gain
- Somnolence
- Activation of Mania or Hypomania
- Seizures
- Elevated Cholesterol and Triglycerides
- Hyponatremia
- Transaminase Elevations
- Discontinuation Syndrome
- Use in Patients with Concomitant Illness
(mirtazapine)Tablets
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.
(mirtazapine)Tablets
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.