Brand Name
Revlimid
Generic Name
Lenalidomide
View Brand Information FDA approval date: December 27, 2005
Classification: Thalidomide Analog
Form: Capsule
What is Revlimid (Lenalidomide)?
IND ICATIONS AND USAGE Lenalidomide is a thalidomide analogue indicated for the treatment of adult patients with: Multiple myeloma , in combination with dexamethasone.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Revlimid (Lenalidomide)
WARNING: EMBRYO-FETAL TOXICITY, HEMATOLOGIC TOXICITY, and VENOUS and ARTERIAL THROMBOEMBOLISM
Embryo-Fetal Toxicity
Do not use REVLIMID during pregnancy. Lenalidomide, a thalidomide analogue, caused limb abnormalities in a developmental monkey study. Thalidomide is a known human teratogen that causes severe life-threatening human birth defects. If lenalidomide is used during pregnancy, it may cause birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting REVLIMID
Information about the Lenalidomide REMS program is available at www.lenalidomiderems.com or by calling the REMS Call Center at 1-888-423-5436.
Hematologic Toxicity (Neutropenia and Thrombocytopenia)
REVLIMID can cause significant neutropenia and thrombocytopenia. Eighty percent of patients with del 5q myelodysplastic syndromes had to have a dose delay/reduction during the major study. Thirty-four percent of patients had to have a second dose delay/reduction. Grade 3 or 4 hematologic toxicity was seen in 80% of patients enrolled in the study. Patients on therapy for del 5q myelodysplastic syndromes should have their complete blood counts monitored weekly for the first 8 weeks of therapy and at least monthly thereafter. Patients may require dose interruption and/or reduction. Patients may require use of blood product support and/or growth factors
Venous and Arterial Thromboembolism
REVLIMID has demonstrated a significantly increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as risk of myocardial infarction and stroke in patients with multiple myeloma who were treated with REVLIMID and dexamethasone therapy. Monitor for and advise patients about signs and symptoms of thromboembolism. Advise patients to seek immediate medical care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling. Thromboprophylaxis is recommended and the choice of regimen should be based on an assessment of the patient’s underlying risks
1DOSAGE FORMS AND STRENGTHS
Capsules:
- 2.5 mg, white and blue-green opaque hard capsules imprinted "REV" on one half and "2.5 mg" on the other half in black ink
- 5 mg, white opaque capsules imprinted "REV" on one half and "5 mg" on the other half in black ink
- 10 mg, blue/green and pale yellow opaque capsules imprinted "REV" on one half and "10 mg" on the other half in black ink
- 15 mg, powder blue and white opaque capsules imprinted "REV" on one half and "15 mg" on the other half in black ink
- 20 mg, powder blue and blue-green opaque hard capsules imprinted "REV" on one half and "20 mg" on the other half in black ink
- 25 mg, white opaque capsules imprinted "REV" on one half and "25 mg" on the other half in black ink
2ADVERSE REACTIONS
The following clinically significant adverse reactions are described in detail in other sections of the prescribing information:
- Embryo-Fetal Toxicity
- Hematologic Toxicity
- Venous and Arterial Thromboembolism
- Increased Mortality in Patients with CLL
- Second Primary Malignancies
- Increased Mortality in Patients with MM When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone
- Hepatotoxicity
- Severe Cutaneous Reactions
- Tumor Lysis Syndrome
- Tumor Flare Reactions
- Impaired Stem Cell Mobilization
- Thyroid Disorders
- Early Mortality in Patients with MCL
- Hypersensitivity
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
2.2Postmarketing Experience
The following adverse drug reactions have been identified from the worldwide post-marketing experience with REVLIMID. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
Endocrine disorders: Hypothyroidism, hyperthyroidism
Hepatobiliary disorders: Hepatic failure (including fatality), toxic hepatitis, cytolytic hepatitis, cholestatic hepatitis, mixed cytolytic/cholestatic hepatitis, transient abnormal liver laboratory tests
Immune system disorders: Angioedema, anaphylaxis, acute graft-versus-host disease (following allogeneic hematopoietic transplant), solid organ transplant rejection
Infections and infestations: Viral reactivation (such as hepatitis B virus and herpes zoster), progressive multifocal leukoencephalopathy (PML)
Neoplasms benign, malignant and unspecified (including cysts and polyps): Tumor lysis syndrome, tumor flare reaction
Respiratory, thoracic and mediastinal disorders: Pneumonitis
Skin and subcutaneous tissue disorders: Stevens-Johnson Syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS)
3OVERDOSAGE
There is no specific experience in the management of REVLIMID overdose in patients with MM, MDS, MCL, FL, or MZL. In dose-ranging studies in healthy subjects, some were exposed to up to 200 mg (administered 100 mg BID) and in single-dose studies, some subjects were exposed to up to 400 mg. Pruritus, urticaria, rash, and elevated liver transaminases were the primary reported AEs. In clinical trials, the dose-limiting toxicity was neutropenia and thrombocytopenia.
4DESCRIPTION
REVLIMID, a thalidomide analogue, is an immunomodulatory agent with antiangiogenic and antineoplastic properties. The chemical name is 3-(4-amino-1-oxo 1,3-dihydro-2
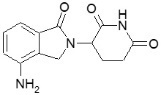
3-(4-amino-1-oxo 1,3-dihydro-2
The empirical formula for lenalidomide is C
Lenalidomide is an off-white to pale-yellow solid powder. It is soluble in organic solvent/water mixtures, and buffered aqueous solvents. Lenalidomide is more soluble in organic solvents and low pH solutions. Solubility was significantly lower in less acidic buffers, ranging from about 0.4 to 0.5 mg/ml. Lenalidomide has an asymmetric carbon atom and can exist as the optically active forms S(-) and R(+), and is produced as a racemic mixture with a net optical rotation of zero.
REVLIMID is available in 2.5 mg, 5 mg, 10 mg, 15 mg, 20 mg and 25 mg capsules for oral administration. Each capsule contains lenalidomide as the active ingredient and the following inactive ingredients: lactose anhydrous, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The 5 mg and 25 mg capsule shell contains gelatin, titanium dioxide and black ink. The 2.5 mg and 10 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink. The 15 mg capsule shell contains gelatin, FD&C blue #2, titanium dioxide and black ink. The 20 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink.
5REFERENCES
1. OSHA Hazardous Drugs.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved Patient labeling (Medication Guide)
7Medication Guide
REVLIMID
(lenalidomide) capsules
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: MAR 2023
8PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 59572-402-28
Revlimid
(lenalidomide) Capsules
2.5 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx Only
28 Capsules
9PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
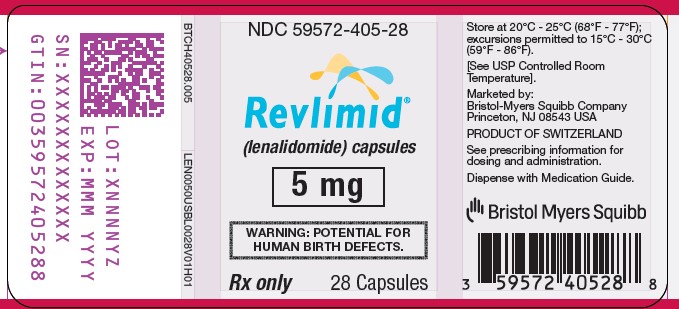
NDC 59572-405-28
Revlimid
(lenalidomide) Capsules
5 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx Only
28 Capsules
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 59572-410-28
Revlimid
(lenalidomide) Capsules
10 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx Only
28 Capsules

11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 59572-415-21
Revlimid
(lenalidomide) Capsules
15 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx Only
21 Capsules

12PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
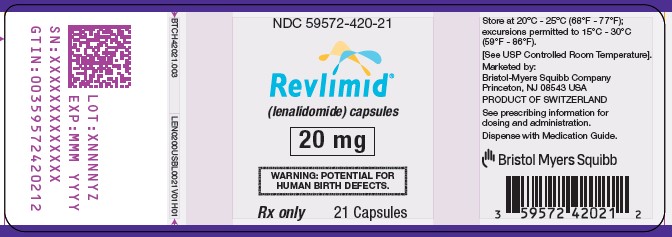
NDC 59572-420-21
Revlimid
(lenalidomide) Capsules
20 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx Only
21 Capsules
13PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 59572-425-21
Revlimid
(lenalidomide) Capsules
25 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx Only
21 Capsules
