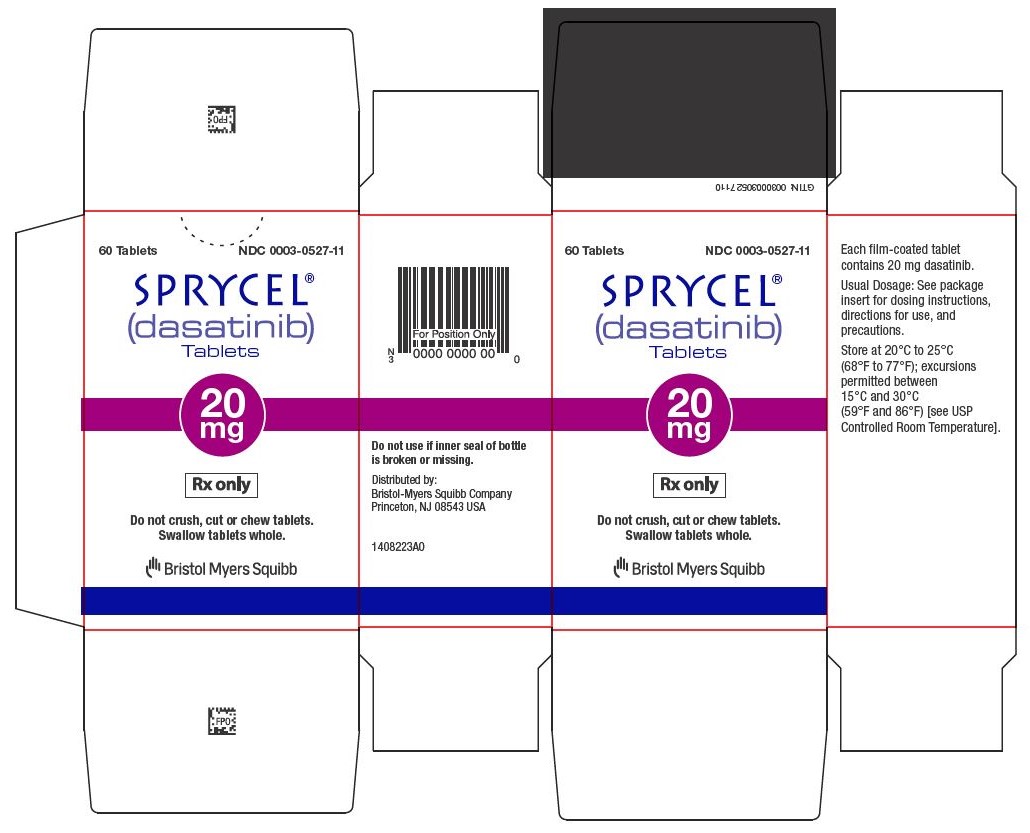
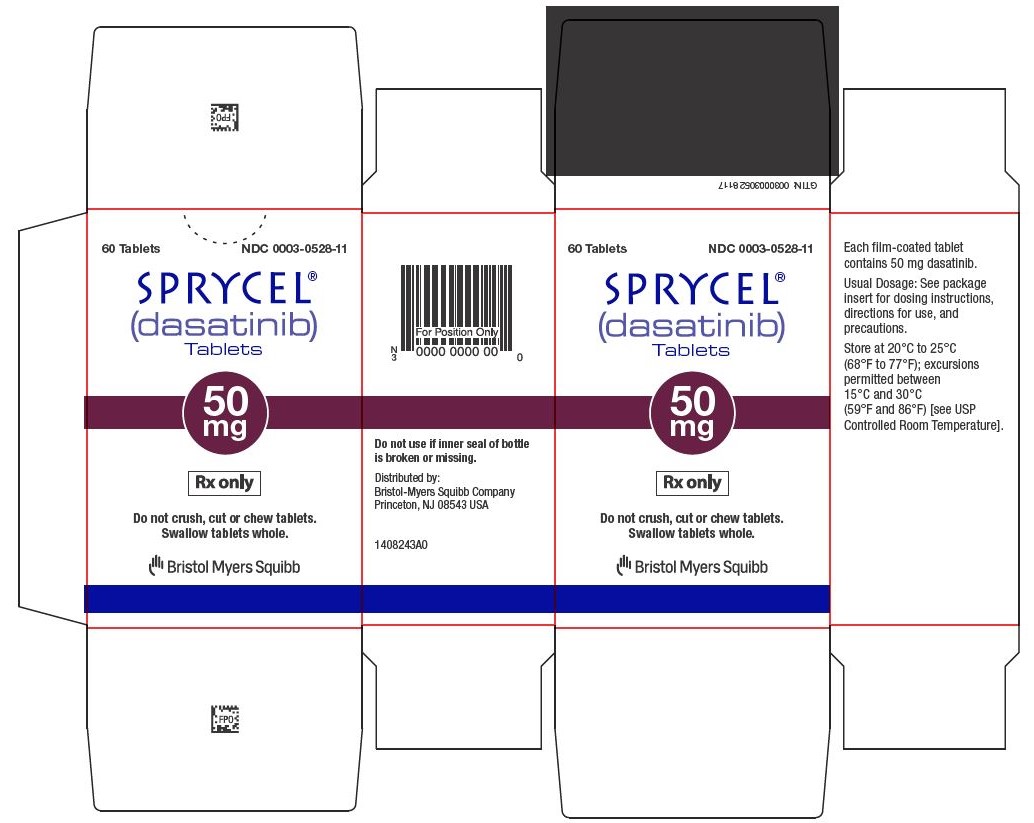
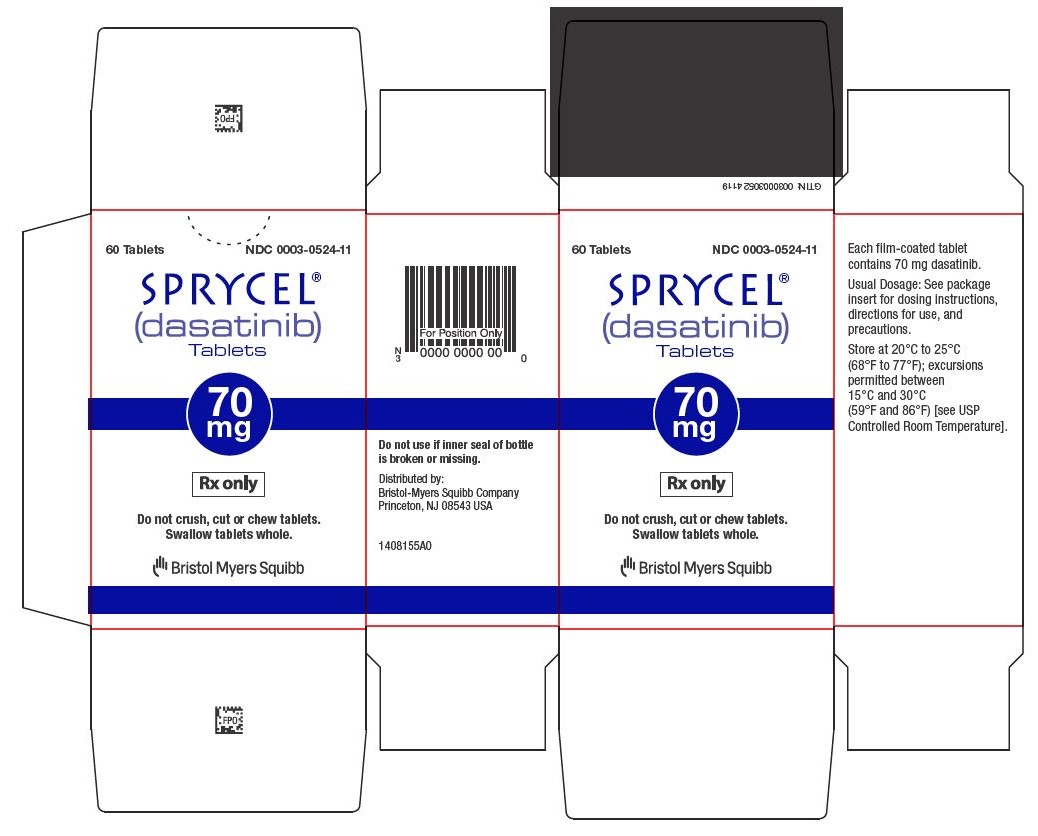
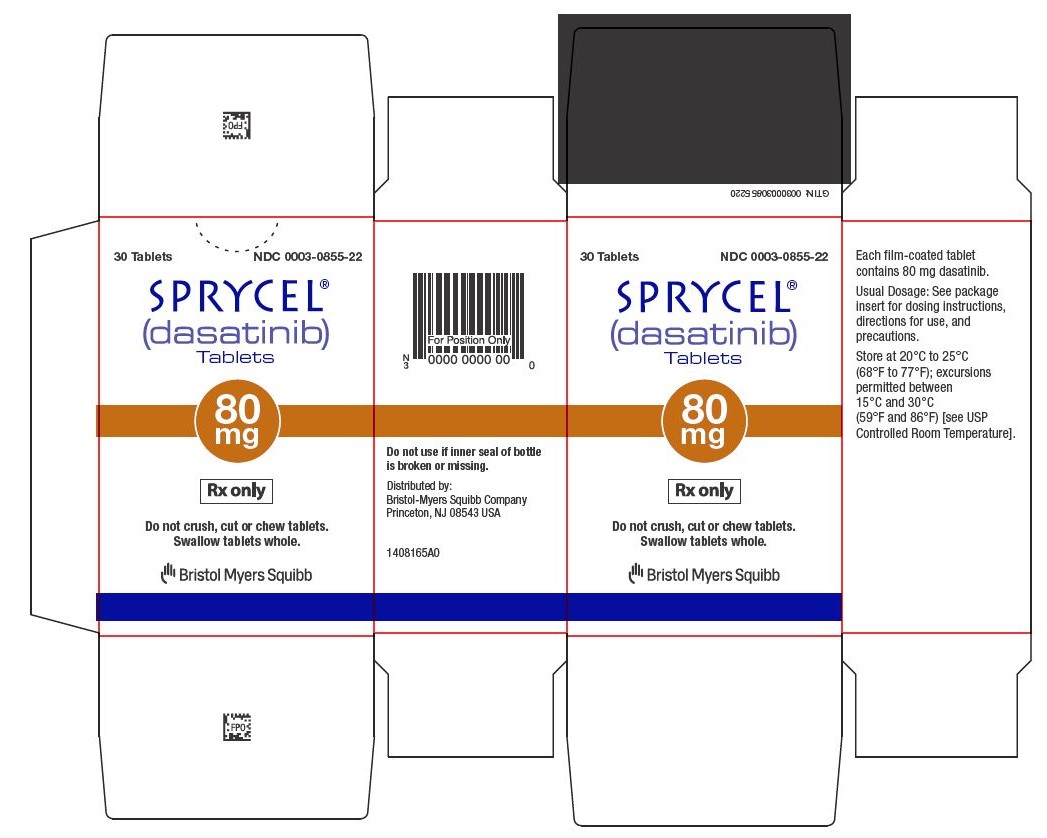
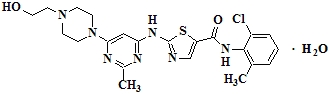
Dasatinib
What is Sprycel (Dasatinib)?
Receiving a leukemia diagnosis can be overwhelming. For many people, it brings uncertainty about treatment, recovery, and what life will look like moving forward. Sprycel (dasatinib) is a medication that gives patients with certain types of leukemia a powerful tool for controlling their disease and reclaiming stability in their lives.
Sprycel is an oral chemotherapy medicine classified as a tyrosine kinase inhibitor (TKI). It is primarily used to treat chronic myeloid leukemia (CML) and Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) in both adults and children. These are cancers of the white blood cells that result from genetic changes causing uncontrolled cell growth.
Approved by the U.S. Food and Drug Administration (FDA) in 2006, Sprycel is considered one of the cornerstone targeted therapies for these conditions. Unlike traditional chemotherapy, it specifically targets the abnormal proteins driving leukemia growth, which allows for effective disease control with fewer generalized side effects.
What does Sprycel do?
Sprycel is used to treat:
- Chronic myeloid leukemia (CML): a slow-growing bone marrow cancer that produces too many white blood cells.
- Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL): a faster-growing leukemia with a specific genetic abnormality that drives cancer cell growth.
Both diseases involve the presence of an abnormal gene called the Philadelphia chromosome, which produces a faulty protein that signals cells to multiply uncontrollably. Sprycel blocks this signal, helping to slow or stop cancer progression.
For many patients, this treatment has transformed leukemia from a life-threatening disease into a manageable chronic condition. Clinical studies have shown that dasatinib can cause deep and durable remissions, significantly improving survival rates and quality of life (NIH, 2024). Some patients even achieve treatment-free remission under careful medical supervision after sustained response.
How does Sprycel work?
Sprycel works by targeting and blocking an enzyme called BCR-ABL, a type of tyrosine kinase produced by the Philadelphia chromosome. This enzyme acts like a stuck accelerator pedal, continuously signaling the bone marrow to produce immature white blood cells that crowd out healthy ones.
Dasatinib attaches to the BCR-ABL enzyme and turns off this overactive signal, helping restore normal blood cell production. It also blocks other related tyrosine kinases involved in cancer growth, giving it a broader range of action than some older TKIs.
In simpler terms, Sprycel stops leukemia cells from growing and multiplying while allowing healthy blood cells to recover. Clinically, this targeted approach matters because it minimizes damage to noncancerous cells, leading to more tolerable long-term therapy compared with traditional chemotherapy.
Patients typically begin to see improvement in blood counts within weeks, and the drug’s continued use helps maintain remission and prevent relapse.
Sprycel side effects
While Sprycel is generally well tolerated, it can cause side effects that vary in intensity from person to person. Most are manageable with monitoring and supportive care.
Common side effects include:
- Headache or fatigue
- Diarrhea or stomach discomfort
- Fluid retention (swelling around the ankles or eyes)
- Skin rash or mild itching
- Muscle or joint pain
Serious side effects (less common) may include:
- Shortness of breath or chest discomfort (possible fluid buildup around the lungs)
- Low blood cell counts, leading to fatigue, bruising, or increased infection risk
- Irregular heartbeat or changes in heart rhythm
- Severe bleeding or unusual bruising
- Liver function changes
Sprycel patients need regular blood tests (white/red cells, platelets, liver function) and heart monitoring (especially with prior heart disease).
Use with caution for severe liver issues, heart rhythm problems, or dasatinib allergies. Avoid grapefruit to prevent increased Sprycel levels and side effects.
Seek immediate medical attention if you experience chest pain, sudden shortness of breath, or signs of severe bleeding.
Sprycel dosage
Sprycel comes in tablet form and is taken by mouth once daily, with or without food. Patients should swallow the tablets whole and avoid crushing or breaking them.
The exact dose depends on the type of leukemia, age, body weight (in children), and individual response to treatment. Doctors may adjust the dose based on how well the cancer responds and whether side effects occur.
Physicians monitor blood and liver function due to Sprycel’s effects, adjusting doses as needed. Swallowing difficulties may allow alternative administration. While older adults and those with existing conditions need closer observation, the medication is generally effective across ages.
Does Sprycel have a generic version?
Yes. Dasatinib, the active ingredient in Sprycel, is available as a generic medication in the United States and many other countries. The FDA has approved several generic versions, which are considered bioequivalent, meaning they provide the same strength, effectiveness, and safety as the brand-name drug.
Generic dasatinib, a more affordable and accessible alternative to brand-name Sprycel, offers identical cancer-fighting benefits. Both are available in various tablet strengths for tailored dosing.
Conclusion
Sprycel (dasatinib) represents a major advancement in the treatment of chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia. By targeting the abnormal protein driving these cancers, it helps patients achieve remission, maintain stable blood counts, and live longer, healthier lives.
With medical supervision, Sprycel is a safe and effective long-term treatment for leukemia, offering improved quality of life with manageable side effects. Open communication with the healthcare team, reporting new symptoms, and adhering to testing schedules are crucial. Sprycel transforms leukemia from a devastating diagnosis into a treatable, manageable condition.
References
- U.S. Food and Drug Administration (FDA). (2024). Sprycel (dasatinib) prescribing information. Retrieved from https://www.accessdata.fda.gov
- MedlinePlus. (2024). Dasatinib: Drug information and usage guide. National Library of Medicine. Retrieved from https://medlineplus.gov
- Mayo Clinic. (2024). Dasatinib (oral route) description and precautions. Retrieved from https://www.mayoclinic.org
- National Institutes of Health (NIH). (2024). Tyrosine kinase inhibitors in leukemia treatment. Retrieved from https://www.nih.gov
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase.
- chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib.
- Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior therapy.
- Ph+ CML in chronic phase.
- newly diagnosed Ph+ ALL in combination with chemotherapy.
- Myelosuppression
- Bleeding-related events
- Fluid retention
- Cardiovascular toxicity
- Pulmonary arterial hypertension
- QT prolongation
- Severe dermatologic reactions
- Tumor lysis syndrome
- Effects on growth and development in pediatric patients
- Hepatotoxicity
Revised: February 2023