Brand Name
Vyzulta
Generic Name
Latanoprostene Bunod
View Brand Information FDA approval date: November 02, 2017
Form: Solution
What is Vyzulta (Latanoprostene Bunod)?
VYZULTA ®
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Vyzulta (latanoprostene bunod)
1INDICATIONS AND USAGE
VYZULTA
2DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the conjunctival sac of the affected eye(s) once daily in the evening.
If VYZULTA is to be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure, administer each drug product at least five (5) minutes apart.
3DOSAGE FORMS AND STRENGTHS
VYZULTA is a topical ophthalmic solution containing latanoprostene bunod, 0.24 mg/mL.
4CONTRAINDICATIONS
None.
5ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Pigmentation
- Eyelash Changes
- Intraocular Inflammation
- Macular Edema
- Bacterial Keratitis
- Use with Contact Lens
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VYZULTA was evaluated in 811 patients in 2 controlled clinical trials of up to 12 months duration. The most common ocular adverse reactions observed in patients treated with latanoprostene bunod were: conjunctival hyperemia (6%), eye irritation (4%), eye pain (3%), and instillation site pain (2%). Approximately 0.6% of patients discontinued therapy due to ocular adverse reactions including ocular hyperemia, conjunctival irritation, eye irritation, eye pain, conjunctival edema, vision blurred, punctate keratitis and foreign body sensation.
6DESCRIPTION
VYZULTA
Its chemical name is 4-(Nitrooxy)butyl (5Z)-7-{(1R,2R,3R,5S)-3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl}hept-5-enoate. Its molecular formula is C
Its chemical structure is:
Figure 1
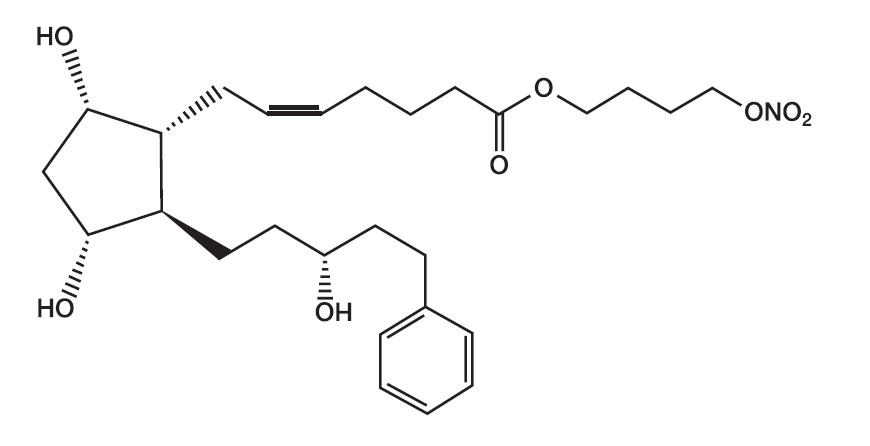
Latanoprostene bunod is a colorless to yellow oil.
7CLINICAL STUDIES
In clinical studies up to 12 months duration, patients with open-angle glaucoma or ocular hypertension with average baseline intraocular pressures (IOPs) of 26.7 mmHg, the IOP-lowering effect of VYZULTA (latanoprostene bunod ophthalmic solution) 0.024% once daily (in the evening) was up to 7 to 9 mmHg.
8HOW SUPPLIED/STORAGE AND HANDLING
VYZULTA
2.5 mL fill in a 4 mL white container - NDC 24208-504-02
5 mL fill in a 7.5 mL natural container - NDC 24208-504-05
Storage:Unopened bottle should be stored refrigerated at 2°C to 8°C (36°F to 46°F). Once a bottle is opened it may be stored at 2°C to 25°C (36°F to 77°F) for 8 weeks.
During shipment, bottles may be maintained at temperatures up to 40°C (104°F) for a period not exceeding 14 days.
Protect from light. Protect from freezing.
9PATIENT COUNSELING INFORMATION
- Potential for Pigmentation
- Patients should be advised about the potential for increased brown pigmentation of the iris, which may be permanent. Patients should also be informed about the possibility of eyelid skin darkening, which is usually reversible after discontinuation of VYZULTA.
- Potential for Eyelash Changes
- Patients should also be informed of the possibility of eyelash and vellus hair changes in the treated eye during treatment with VYZULTA. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
- Handling the Container
- Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
- When to Seek Physician Advice
- Advise patients that if they develop a new ocular condition (e.g., trauma or infection), experience a sudden decrease in visual acuity, have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of VYZULTA.
- Use with Contact Lenses
- Contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of VYZULTA.
- Use with Other Ophthalmic Drugs
- If more than one topical ophthalmic drug is being used, the drugs should be administered with at least five (5) minutes between applications.
Patented. See https://patents.bausch.com for US patent information.
VYZULTA is a trademark of Bausch & Lomb Incorporated or its affiliates.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
© 2024 Bausch & Lomb Incorporated or its affiliates
9612404 (Folded)
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC24208-504-05
VYZULTA
(latanoprostene
bunod
ophthalmic solution)
0.024%
Sterile
FOR TOPICAL OPHTHALMIC USE
Rx only
5 mL