Generic Name
Ursodiol
Brand Names
Urso 250, Reltone, Urso
FDA approval date: December 31, 1987
Classification: Bile Acid
Form: Tablet, Capsule
What is Urso 250 (Ursodiol)?
Ursodiol capsules are indicated for patients with radiolucent, noncalcified gallbladder stones <20 mm in greatest diameter in whom elective cholecystectomy would be undertaken except for the presence of increased surgical risk due to systemic disease, advanced age, idiosyncratic reaction to general anesthesia, or for those patients who refuse surgery. Safety of use of ursodiol beyond 24 months is not established. Ursodiol capsules are indicated for the prevention of gallstone formation in obese patients experiencing rapid weight loss.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Urso 250 (Ursodiol)
1INDICATIONS AND USAGE
URSO 250 and URSO Forte (ursodiol) tablets are indicated for the treatment of patients with primary biliary cholangitis (PBC).
2DOSAGE FORMS AND STRENGTHS
- URSO 250: 250 mg tablet
- URSO Forte: 500 mg scored tablet
3CONTRAINDICATIONS
Patients with complete biliary obstruction and known hypersensitivity or intolerance to ursodiol or any of the components of the formulation.
4OVERDOSAGE
There have been no reports of accidental or intentional overdosage with ursodiol. Single oral doses of ursodiol at 10 g/kg in mice and dogs, and 5 g/kg in rats were not lethal. A single oral dose of ursodiol at 1.5 g/kg was lethal in hamsters. Symptoms of acute toxicity were salivation and vomiting in dogs, and ataxia, dyspnea, ptosis, agonal convulsions and coma in hamsters.
5DESCRIPTION
URSO 250 (ursodiol, 250 mg) is available as a film-coated tablet for oral administration. URSO Forte (ursodiol, 500 mg) is available as a scored film-coated tablet for oral administration.
Ursodiol (ursodeoxycholic acid, UDCA) is a naturally occurring bile acid found in small quantities in normal human bile and in larger quantities in the biles of certain species of bears. It is a bitter-tasting white powder consisting of crystalline particles freely soluble in ethanol and glacial acetic acid, slightly soluble in chloroform, sparingly soluble in ether, and practically insoluble in water. The chemical name of ursodiol is 3α,7ß-dihydroxy-5ß-cholan-24-oic (C
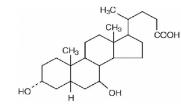
Inactive ingredients: microcrystalline cellulose, povidone, sodium starch glycolate, magnesium stearate, ethylcellulose, dibutyl sebacate, carnauba wax, hydroxypropyl methylcellulose, PEG 3350, PEG 8000, cetyl alcohol, sodium lauryl sulfate and hydrogen peroxide.
6PATIENT COUNSELING INFORMATION
Enteroliths in Patients with Risk for Intestinal Stenosis or Stasis
Advise patients or their caretaker(s) to notify their healthcare provider if they experience obstructive gastrointestinal symptoms due to the risk of enteroliths
Drug Interactions
Patients should be informed that absorption of URSO 250 and URSO Forte may be reduced if they are taking bile acid sequestering agents, such as cholestyramine and colestipol, aluminum-based antacids, or drugs known to alter the metabolism of cholesterol
Distributed by:
Allergan USA, Inc.
Madison, NJ 07940
www.allergan.com
Allergan USA, Inc.
Madison, NJ 07940
www.allergan.com

URSO 250
v4.0USPI785
7Principal Display Panel
- Urso 250 Bottle Label
NDC 58914-785-10
100 tablets
Rx only
Urso® 250
(Ursodiol Tablets, USP) 250 mg
Allergan™
NDC 58914-785-10
100 tablets
Rx only
Urso® 250
(Ursodiol Tablets, USP) 250 mg
Allergan™

8Principal Display Panel
- Urso 500 Bottle Label
NDC 58914-790-10
100 tablets
Urso Forte®
(Ursodiol Tablets, USP)
500 mg
Rx only
For the treatment of
Primary Biliary Cirrhosis
Allergan™
NDC 58914-790-10
100 tablets
Urso Forte®
(Ursodiol Tablets, USP)
500 mg
Rx only
For the treatment of
Primary Biliary Cirrhosis
Allergan™
