Brand Name
Aptiom
Generic Name
Eslicarbazepine Acetate
View Brand Information FDA approval date: April 07, 2014
Form: Tablet, Kit
What is Aptiom (Eslicarbazepine Acetate)?
Eslicarbazepine acetate tablets are indicated for the treatment of partial-onset seizures in patients 4 years of age and older. Eslicarbazepine acetate tablets are indicated for the treatment of partial-onset seizures in patients 4 years of age and older.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Aptiom (ESLICARBAZEPINE ACETATE)
1INDICATIONS AND USAGE
APTIOM is indicated for the treatment of partial-onset seizures in patients 4 years of age and older.
2DOSAGE FORMS AND STRENGTHS
APTIOM tablets are available in the following shapes and color (
3CONTRAINDICATIONS
APTIOM is contraindicated in patients with a hypersensitivity to eslicarbazepine acetate or oxcarbazepine
4ADVERSE REACTIONS
The following adverse reactions are described in more detail in the
- Suicidal Behavior and Ideation
- Serious Dermatologic Reactions
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Anaphylactic Reactions and Angioedema
- Hyponatremia
- Neurological Adverse Reactions
- Drug Induced Liver Injury
- Abnormal Thyroid Function Tests
- Pancytopenia, Agranulocytosis, and Leukopenia
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of APTIOM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Hematologic and Lymphatic Systems: leukopenia, agranulocytosis, thrombocytopenia, megaloblastic anemia, and pancytopenia
Metabolism and Nutrition Disorders: syndrome of inappropriate antidiuretic hormone secretion (SIADH)
5DESCRIPTION
The chemical name of APTIOM (eslicarbazepine acetate) is (S)-10-Acetoxy-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide. APTIOM is a dibenz[b,f]azepine-5-carboxamide derivative. Its molecular formula is C
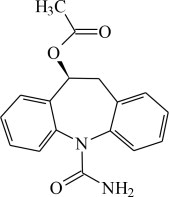
APTIOM is a white to off-white, odorless crystalline solid. It is insoluble in hexane, very slightly soluble in aqueous solvents and soluble in organic solvents such as acetone, acetonitrile, and methanol.
Each APTIOM tablet contains 200 mg, 400 mg, 600 mg or 800 mg of eslicarbazepine acetate and the following inactive ingredients: croscarmellose sodium, magnesium stearate, and povidone.
6REFERENCES
French JA, Wang S, Warnock B, Temkin N.
7PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
Inform patients and caregivers of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking APTIOM. Instruct patients and caregivers that APTIOM should only be taken as prescribed.


