Brand Name
Valcyte
Generic Name
Valganciclovir
View Brand Information FDA approval date: March 29, 2001
Classification: Cytomegalovirus Nucleoside Analog DNA Polymerase Inhibitor
Form: Tablet, Powder, For
What is Valcyte (Valganciclovir)?
Valganciclovir tablet is a deoxynucleoside analogue cytomegalovirus DNA polymerase inhibitor indicated for: Adult Patients.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Valcyte (valganciclovir)
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with VALCYTE
- Impairment of Fertility: Based on animal data and limited human data, VALCYTE may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females
- Fetal Toxicity: Based on animal data, VALCYTE has the potential to cause birth defects in humans
- Mutagenesis and Carcinogenesis: Based on animal data, VALCYTE has the potential to cause cancers in humans
1DOSAGE FORMS AND STRENGTHS
- VALCYTE tablets: 450 mg, pink, film-coated convex oval tablets with "VGC" on one side and "450" on the other side.
- VALCYTE for oral solution: 50 mg per mL, supplied as a white to slightly yellow powder for constitution, forming a colorless to brownish yellow tutti-frutti flavored solution. Available in glass bottles containing approximately 100 mL of solution after constitution.
2CONTRAINDICATIONS
VALCYTE is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valganciclovir, ganciclovir, or any component of the formulation
3ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Hematologic Toxicity
- Acute Renal Failure
- Impairment of Fertility
- Fetal Toxicity
- Mutagenesis and Carcinogenesis
The most common adverse reactions and laboratory abnormalities reported in at least one indication by greater than or equal to 20% of adult patients treated with VALCYTE tablets are diarrhea, pyrexia, fatigue, nausea, tremor, neutropenia, anemia, leukopenia, thrombocytopenia, headache, insomnia, urinary tract infection, and vomiting. The most common reported adverse reactions and laboratory abnormalities reported in greater than or equal to 20% of pediatric solid organ transplant recipients treated with VALCYTE for oral solution or tablets are diarrhea, pyrexia, upper respiratory tract infection, urinary tract infection, vomiting, neutropenia, leukopenia, and headache.
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
Valganciclovir, a prodrug of ganciclovir, is rapidly converted to ganciclovir after oral administration. Adverse reactions known to be associated with ganciclovir usage can therefore be expected to occur with VALCYTE.
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of VALCYTE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. As VALCYTE is rapidly and extensively converted to ganciclovir, any adverse reactions associated with ganciclovir might also occur with valganciclovir.
- Anaphylactic reaction
- Agranulocytosis
- Granulocytopenia
In general, the adverse reactions reported during the postmarketing use of VALCYTE were similar to those identified during the clinical trials.
4DRUG INTERACTIONS
In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, drug-drug interactions associated with ganciclovir will be expected for VALCYTE. Drug-drug interaction studies with ganciclovir were conducted in patients with normal renal function. Following concomitant administration of VALCYTE and other renally excreted drugs, patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered drug. Therefore, these patients should be closely monitored for toxicity of ganciclovir and the coadministered drug.
Established and other potentially significant drug interactions conducted with ganciclovir are listed in
5DESCRIPTION
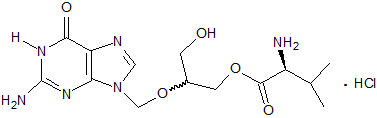
VALCYTE contains valganciclovir hydrochloride (valganciclovir HCl), a hydrochloride salt of the L-valyl ester of ganciclovir that exists as a mixture of two diastereomers. Ganciclovir is a synthetic guanine derivative active against CMV.
VALCYTE is available as a 450 mg tablet for oral administration. Each tablet contains 496.3 mg of valganciclovir HCl (corresponding to 450 mg of valganciclovir), and the inactive ingredients microcrystalline cellulose, povidone K-30, crospovidone and stearic acid. The film-coat applied to the tablets contains Opadry Pink
VALCYTE is also available as a powder for oral solution, which when constituted with water as directed contains 50 mg/mL valganciclovir free base. The inactive ingredients of VALCYTE for oral solution are sodium benzoate, fumaric acid, povidone K-30, sodium saccharin, mannitol and tutti-frutti flavoring.
Valganciclovir HCl is a white to off-white crystalline powder with a molecular formula of C
The chemical structure of valganciclovir HCl is:
All doses in this insert are specified in terms of valganciclovir.
6REFERENCES
1. Brion LP, Fleischman AR, McCarton C, Schwartz GJ. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J of Ped 1986: 109(4): 698-707.
2. NIOSH [2014]. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings. By Connor TH, MacKenzie BA, DeBord DG, Trout DB, O'Callaghan JP, Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2014-138 (Supersedes 2012-150).
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
8Instructions for Use VALCYTE (Val-site) (valganciclovir) for oral solution
Be sure that you read, and that you understand and follow these instructions carefully to ensure proper dosing of the oral solution.
Important:
- Avoid contact with your skin or eyes. If you come in contact with the contents of the oral solution, wash your skin well with soap and water or rinse your eyes well with plain water.
- Do not use VALCYTE for oral solution after the discard date on the bottle.
- Always use the oral dispenser provided to give or take a dose of VALCYTE for oral solution.
- Call your pharmacist if your oral dispenser is lost or damaged, and they will tell you how to continue to give or take a dose of VALCYTE for oral solution.
- Ask your healthcare provider or pharmacist to show you how to measure your prescribed dose.
To take a dose of VALCYTE for oral solution, you will need the bottle of medicine and an oral dispenser provided with the medicine (see
How should I store VALCYTE for oral solution?
- Store solution in the refrigerator at 36°F to 46°F (2°C to 8°C) for no longer than 49 days.
- Do not freeze.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
VALCYTE is a registered trademark of CHEPLAPHARM Arzneimittel GmbH.
Distributed by:
Licensed by:
Revised: 04/2023
For more information call 1-866-946-3684.
© 2023 CHEPLAPHARM Arzneimittel GmbH. All rights reserved.
9PRINCIPAL DISPLAY PANEL - 450 mg Bottle Carton
NDC 61269-480-60
Valcyte
450 mg
DO NOT BREAK OR CRUSH TABLETS
CAUTION: Strict adherence to dosage
60 tablets
CHEPLAPHARM

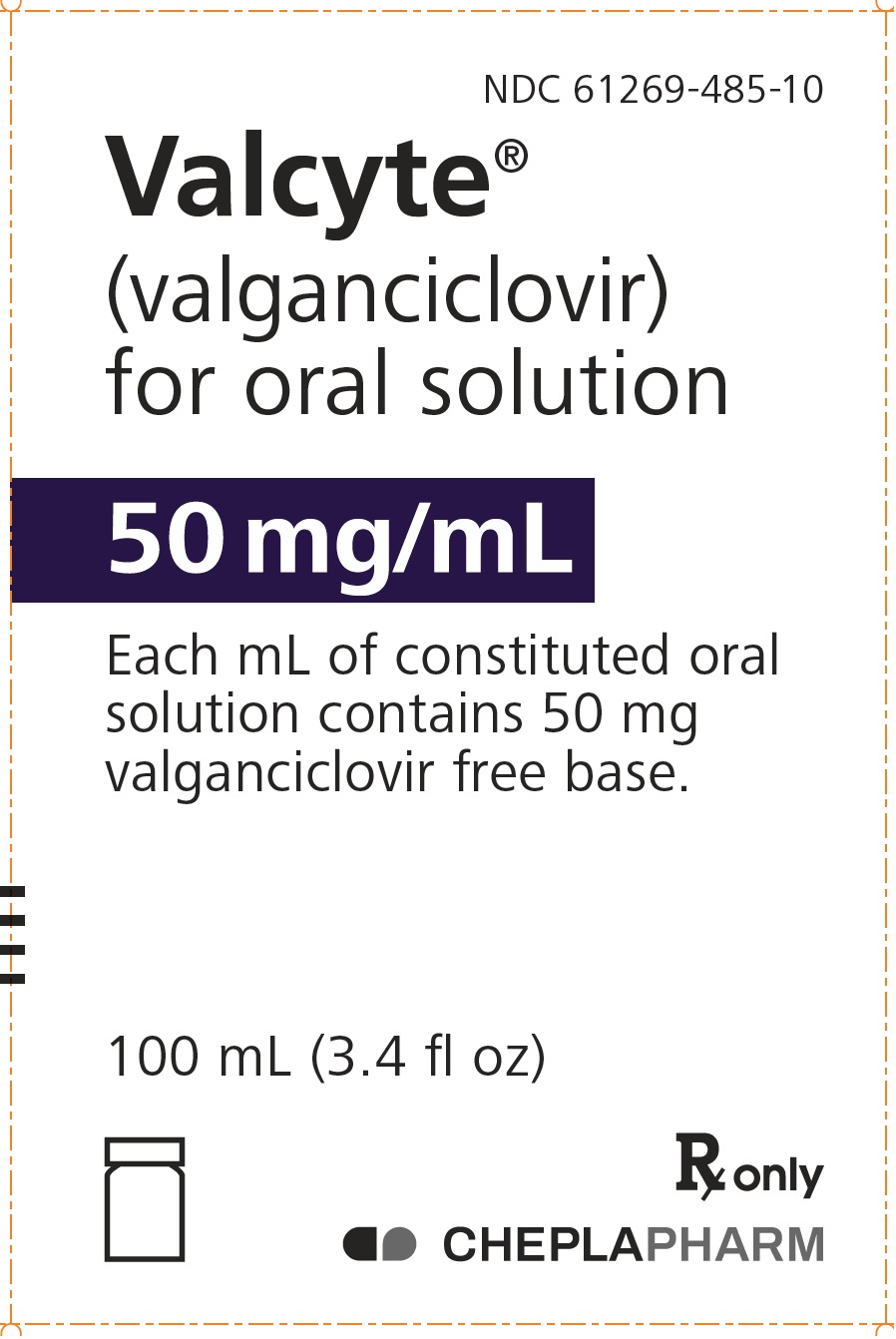
10PRINCIPAL DISPLAY PANEL - 100 mL Bottle Carton
NDC 61269-485-10
Valcyte
50 mg/mL
Each mL of constituted oral
100 mL (3.4 fl oz)
Rx only
CHEPLAPHARM