Brand Name
Fycompa
Generic Name
Perampanel
View Brand Information FDA approval date: October 22, 2012
Classification: Noncompetitive AMPA Glutamate Receptor Antagonist
Form: Tablet, Suspension
What is Fycompa (Perampanel)?
Perampanel tablets, a non-competitive AMPA glutamate receptor antagonist, are indicated for: Treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy 4 years of age and older.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Fycompa (perampanel)
WARNING: SERIOUS PSYCHIATRIC AND BEHAVIORAL REACTIONS
- Serious or life-threatening psychiatric and behavioral adverse reactions including aggression, hostility, irritability, anger, and homicidal ideation and threats have been reported in patients taking FYCOMPA (5.1).
- These reactions occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression (5.1).
- Advise patients and caregivers to contact a healthcare provider immediately if any of these reactions or changes in mood, behavior, or personality that are not typical for the patient are observed while taking FYCOMPA or after discontinuing FYCOMPA (5.1).
- Closely monitor patients particularly during the titration period and at higher doses (5.1).
- FYCOMPA should be reduced if these symptoms occur and should be discontinued immediately if symptoms are severe or are worsening (5.1).
1DOSAGE FORMS AND STRENGTHS
Tablets
- 2 mg tablets: orange, round, debossed with “2” on one side and “Є 275” on the other.
- 4 mg tablets: red, round, debossed with “4” on one side and “Є 277” on the other.
- 6 mg tablets: pink, round, debossed with “6” on one side and “Є 294” on the other.
- 8 mg tablets: purple, round, debossed with “8” on one side and “Є 295” on the other.
- 10 mg tablets: green, round, debossed with “10” on one side and “Є 296” on the other.
- 12 mg tablets: blue, round, debossed with “12” on one side and “Є 297” on the other.
Oral Suspension
0.5 mg/mL white to off-white opaque liquid suspension for oral administration.
0.5 mg/mL white to off-white opaque liquid suspension for oral administration.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Serious Psychiatric and Behavioral Reactions
- Suicidal Behavior and Ideation
- Neurologic Effects
- Falls
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
3.1Clinical TrialsExperience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Partial-Onset Seizures
Adult and Adolescent Patients (12 years of age and older)
A total of 1,038 patients receiving FYCOMPA (2, 4, 8, or 12 mg once daily) constituted the safety population in the pooled analysis of the placebo-controlled trials (Studies 1, 2, and 3) in patients with partial-onset seizures. Approximately 51% of patients were female, and the mean age was 35 years.
Adverse Reactions Leading to Discontinuation
In controlled clinical trials (Studies 1, 2, and 3), the rate of discontinuation as a result of an adverse reaction was 3%, 8%, and 19% in patients randomized to receive FYCOMPA at the recommended doses of 4 mg, 8 mg, and 12 mg per day, respectively, and 5% in patients randomized to receive placebo
Most Common Adverse Reactions
Table 2 gives the incidence in the controlled clinical trials (Studies 1, 2, and 3) of the adverse reactions that occurred in ≥2% of patients with partial-onset seizures in the FYCOMPA 12 mg dose group and more frequent than placebo (in order of decreasing frequency for the 12 mg dose group).
The most common dose-related adverse reactions in patients receiving FYCOMPA at doses of 8 mg or 12 mg (≥4% and occurring at least 1% higher than the placebo group) included dizziness (36%), somnolence (16%), fatigue (10%), irritability (9%), falls (7%), nausea (7%), ataxia (5%), balance disorder (4%), gait disturbance (4%), vertigo (4%), and weight gain (4%). For almost every adverse reaction, rates were higher on 12 mg and more often led to dose reduction or discontinuation.
Pediatric Patients (4 to <12 years of age)
In two studies in pediatric patients 4 to <12 years of age with epilepsy, a total of 225 patients received FYCOMPA, with 110 patients exposed for at least 6 months, and 21 patients for at least 1 year. Adverse reactions in pediatric patients 4 to <12 years of age were similar to those seen in patients 12 years of age and older.
Primary Generalized Tonic-Clonic Seizures
A total of 81 patients receiving FYCOMPA 8 mg once daily constituted the safety population in the placebo-controlled trial in patients with primary generalized tonic-clonic seizures (Study 4). Approximately 57% of patients were female, and the mean age was 27 years.
In the controlled primary generalized tonic-clonic seizure clinical trial (Study 4), the adverse reaction profile was similar to that noted for the controlled partial-onset seizure clinical trials (Studies 1, 2, and 3).
Table 3 gives the incidence of adverse reactions in patients receiving FYCOMPA 8 mg (≥4% and higher than in the placebo group) in Study 4. The most common adverse reactions in patients receiving FYCOMPA (≥10% and greater than placebo) were dizziness (32%), fatigue (15%), headache (12%), somnolence (11%), and irritability (11%).
The adverse reactions most commonly leading to discontinuation in patients receiving FYCOMPA 8 mg (≥2% and greater than placebo) were vomiting (2%) and dizziness (2%).
Weight Gain
Weight gain has occurred with FYCOMPA.
In controlled partial-onset seizure clinical trials, FYCOMPA-treated adults gained an average of 1.1 kg (2.5 lbs) compared to an average of 0.3 kg (0.7 lbs) in placebo-treated adults with a median exposure of 19 weeks. The percentages of adults who gained at least 7% and 15% of their baseline body weight in FYCOMPA-treated patients were 9.1% and 0.9%, respectively, as compared to 4.5% and 0.2% of placebo-treated patients, respectively. Clinical monitoring of weight is recommended.
Similar increases in weight were also observed in adult and adolescent patients treated with FYCOMPA in the primary generalized tonic-clonic seizure clinical trial.
Elevated triglycerides
Increases in triglycerides have occurred with FYCOMPA use.
Comparison of Sex and Race
No significant sex differences were noted in the incidence of adverse reactions.
Although there were few non-Caucasian patients, no differences in the incidence of adverse reactions compared to Caucasian patients were observed.
3.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of FYCOMPA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.5)]
Psychiatric: Acute psychosis, hallucinations, delusions, paranoia, delirium, confusional state, disorientation, memory impairment [see Warnings and Precautions (5.1)].
4OVERDOSAGE
The highest reported overdose of FYCOMPA was 300 mg. Events reported after FYCOMPA overdose include somnolence, stupor, coma, psychiatric or behavioral reactions, altered mental status, and dizziness or gait disturbances.
There is no available specific antidote to the overdose reactions of FYCOMPA. In the event of overdose, standard medical practice for the management of any overdose should be used. An adequate airway, oxygenation, and ventilation should be ensured; monitoring of cardiac rhythm and vital sign measurement is recommended. A certified poison control center should be contacted for updated information on the management of overdose with FYCOMPA. Due to its long half-life, the reactions caused by FYCOMPA could be prolonged.
5DESCRIPTION
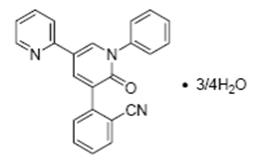
FYCOMPA tablets and oral suspension contain perampanel, a non-competitive AMPA receptor antagonist, as a 4:3 hydrate.
The chemical name of the active ingredient is 2-(1′,6′-dihydro-6′-oxo-1′-phenyl[2,3′-bipyridin]-5′-yl)-benzonitrile, hydrate (4:3).
The molecular formula is C
Tablets
FYCOMPA tablets are round, bi-convex, film-coated tablets containing 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, or 12 mg of perampanel. Tablets contain the following inactive ingredients: lactose monohydrate, low substituted hydroxypropyl cellulose, povidone, microcrystalline cellulose, magnesium stearate, hypromellose, polyethylene glycol, talc, and titanium dioxide. Tablets of different strengths may contain yellow ferric oxide (10 mg and 2 mg), red ferric oxide (2 mg, 4 mg, 6 mg, 8 mg), black ferric oxide (8 mg), and FD&C Blue No. 2 (indigo carmine) aluminum lake (10 mg and 12 mg).
Oral Suspension
FYCOMPA oral suspension is a white to off-white opaque liquid providing perampanel in a concentration of 0.5 mg/mL. The oral suspension contains the following inactive ingredients: sorbitol, microcrystalline cellulose, carboxymethyl-cellulose sodium, poloxamer, simethicone, citric acid, sodium benzoate and purified water.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Administration of Oral Suspension
Advise patients who are prescribed the oral suspension to shake the bottle well before every administration and to use the adaptor and oral dosing syringe provided. Advise patients that a household teaspoon or tablespoon is not an adequate measuring device. Instruct patients to discard any unused FYCOMPA oral suspension remaining 90 days after first opening the bottle
Serious Psychiatric and Behavioral Reactions
Counsel patients, families, and caregivers of patients of the need to monitor for the emergence of anger, aggression, hostility, hallucinations, delusions, confusion, unusual changes in mood, personality, or behavior, and other behavioral symptoms. Advise them to report any such symptoms immediately to their healthcare providers
Suicidal Thinking and Behavior
Counsel patients, their caregivers, and families that AEDs, including FYCOMPA, may increase the risk of suicidal thinking and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers, and families to report behaviors of concern immediately to healthcare providers
Neurologic Effects: Dizziness,Gait Disturbance, Somnolence, and Fatigue
Counsel patients that FYCOMPA may cause dizziness, gait disturbance, somnolence, and fatigue. Advise patients taking FYCOMPA not to drive, operate complex machinery, or engage in other hazardous activities until they have become accustomed to any such effects associated with FYCOMPA
Falls
Counsel patients that FYCOMPA may cause falls and injuries
DRESS/Multi-organ Hypersensitivity
Instruct patients that a fever associated with signs of other organ system involvement (e.g., rash, lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately
Withdrawal of Antiepileptic Drugs
Counsel patients that abrupt discontinuation of FYCOMPA may increase seizure frequency
Contraceptives
Counsel females of reproductive potential that FYCOMPA may decrease efficacy of contraceptives containing levonorgestrel, and advise them to use an additional non-hormonal form of contraception while using FYCOMPA and for a month after discontinuation
Alcohol and Other CNS Depressants
Counsel patients that FYCOMPA may enhance the impairment effects of alcohol. These effects may also be seen if FYCOMPA is taken with other CNS depressants
Missed Doses
Counsel patients that if they miss a dose, they should resume dosing the following day at their prescribed daily dose. Instruct patients to contact their physician if more than one day of dosing is missed.
Controlled Substance
Counsel patients that FYCOMPA is a controlled substance that can be misused and abused
Pregnancy Registry
Advise women who are exposed to FYCOMPA during pregnancy that there is a pregnancy exposure registry that monitors pregnancy outcomes. Encourage these patients to enroll in the NAAED Pregnancy Registry
FYCOMPA
Marketed by Eisai Inc., Nutley, NJ 07110
© 2012-2022 Eisai Inc.