Generic Name
Halobetasol
Brand Names
Bryhali, Lexette, Ultravate
FDA approval date: December 16, 2004
Classification: Corticosteroid
Form: Lotion, Ointment, Cream, Aerosol
What is Bryhali (Halobetasol)?
Halobetasol propionate ointment.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Bryhali (halobetasol propionate)
1INDICATIONS AND USAGE
BRYHALI
2DOSAGE AND ADMINISTRATION
Apply a thin layer of BRYHALI Lotion to affected areas once daily. Rub in gently. Wash hands after each application, unless BRYHALI Lotion is for treatment of the hands.
BRYHALI Lotion treatment beyond 8 weeks is not recommended, and the total dosage should not exceed approximately 50 g per week. Discontinue treatment if control is achieved before 8 weeks. Do not use with occlusive dressings unless directed by a physician.
BRYHALI Lotion should not be used on the face, groin, or in the axillae.
BRYHALI Lotion is not for oral, ophthalmic, or intravaginal use.
3DOSAGE FORMS AND STRENGTHS
Lotion, 0.01%
Each gram of BRYHALI Lotion contains 0.1 mg (0.01%) halobetasol propionate in a white to off-white lotion.
4CONTRAINDICATIONS
None.
5DESCRIPTION
BRYHALI (halobetasol propionate) lotion contains a corticosteroid, halobetasol propionate, as the active ingredient in a white to off-white lotion formulation intended for topical use.
Halobetasol propionate is a synthetic corticosteroid. The chemical name for halobetasol propionate is 21-chloro-6α, 9-difluoro-11β, 17-dihydroxy-16β-methylpregna-1, 4-diene-3, 20 –dione, 17-propionate. Halobetasol propionate is a white to off-white crystalline powder with a molecular weight of 484.96 and a molecular formula of C
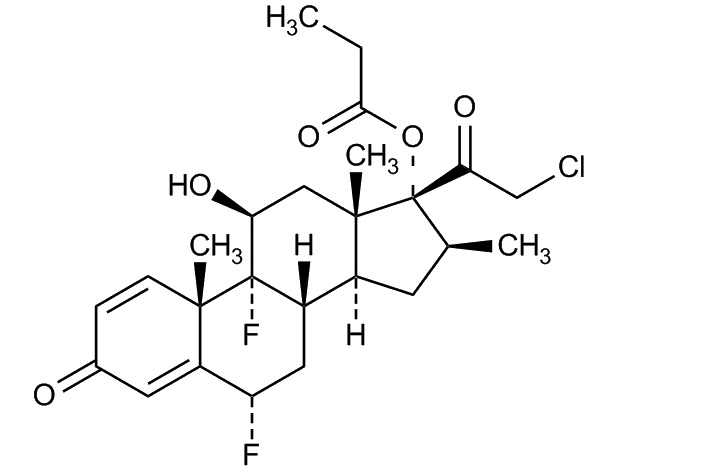
Each gram of BRYHALI Lotion contains 0.1 mg (0.01%) halobetasol propionate in a white to off-white lotion base consisting of carbomer copolymer type B, carbomer homopolymer type A, diethyl sebacate, edetate disodium dihydrate, light mineral oil, methylparaben, propylparaben, purified water, sodium hydroxide, sorbitan monooleate and sorbitol solution, 70%.
6CLINICAL STUDIES
BRYHALI Lotion was evaluated for the treatment of moderate to severe plaque psoriasis in two prospective, multicenter, randomized, double-blind clinical trials (Trial 1 [NCT02514577] and Trial 2 [NCT02515097]). These trials were conducted in 430 subjects 18 years of age and older with moderate to severe plaque psoriasis that covered a body surface area (BSA) between 3% and 12% excluding the face, scalp, palms, soles, axillae, and intertriginous areas. Disease severity was determined by a 5-grade Investigator’s Global Assessment (IGA). Subjects applied BRYHALI Lotion or vehicle to all affected areas once daily for up to 8 weeks. Subjects had a follow-up visit 4 weeks after the end of treatment (Week 12) where safety and efficacy were evaluated.
The primary efficacy endpoint was the proportion of subjects with treatment success at Week 8, where treatment success was defined as at least a 2-grade improvement from baseline in IGA score and an IGA score equating to “clear” or “almost clear”. Table 2 lists the primary efficacy results for Trials 1 and 2. The secondary efficacy endpoints evaluated treatment success sequentially at Weeks 12, 6, 4, and 2. Figure 1 shows the primary and secondary efficacy results over time.
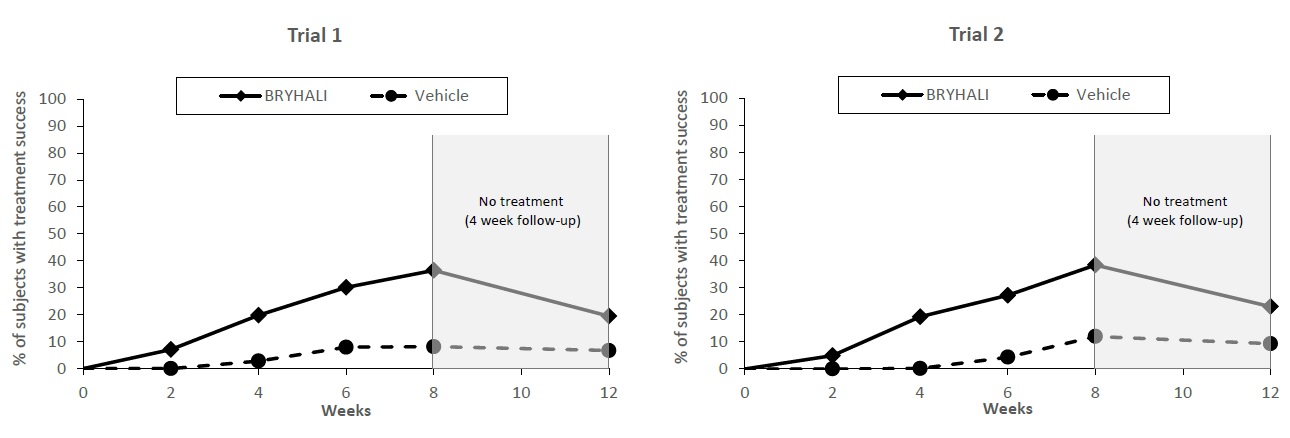
*The treatment difference at Week 2 in Trial 2 was not statistically significant.
7HOW SUPPLIED/STORAGE AND HANDLING
BRYHALI (halobetasol propionate) lotion, 0.01% is a white to off-white lotion supplied in a white aluminum tube as follows:
- 60 g (NDC 0187-0002-60)
- 100 g (NDC 0187-0002-01)
Storage and Handling Conditions
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all administration instructions or all possible adverse or unintended effects.
Advise patients using BRYHALI Lotion of the following information and instructions:
Important Administration Instructions
Instruct patients to discontinue BRYHALI Lotion when psoriasis is controlled. Inform patients that BRYHALI Lotion is to be used as directed by the physician and should not be used for longer than the prescribed time period. Total dosage should not exceed 50 grams per week
Instruct patients to avoid bandaging, wrapping or otherwise occluding the treatment area(s), unless directed by physician. Advise patients to avoid use on the face, groin, or axillae
Inform patients that BRYHALI Lotion is for external use only. Advise patients that BRYHALI Lotion is not for oral, ophthalmic, or intravaginal use
Breastfeeding women should not apply BRYHALI Lotion directly to the nipple and areola to avoid directly exposing the infant
Effects on Endocrine System
BRYHALI Lotion may cause HPA axis suppression. Advise patients that use of topical corticosteroids, including BRYHALI Lotion, may require periodic evaluation for HPA axis suppression. Topical corticosteroids may have other endocrine effects. Concomitant use of multiple corticosteroid-containing products may increase the total systemic exposure to topical corticosteroids
Local Adverse Reactions
Inform patients that BRYHALI Lotion may cause local adverse reactions. These reactions may be more likely to occur with occlusive use or prolonged use of BRYHALI Lotion
Distributed by:Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
U.S. Patent Numbers: 8,809,307 and 10,478,502
BRYHALI is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
TEAR HERE (Patient Information)
9Patient Package Insert
PATIENT INFORMATION
BRYHALI
(halobetasol propionate) Lotion
Important: BRYHALI is for use on the skin only. Do not apply BRYHALI in your mouth, eyes, or vagina.
What is BRYHALI?
BRYHALI is a prescription corticosteroid medicine used on the skin (topical) to treat adults with plaque psoriasis.
It is not known if BRYHALI is safe and effective in children under 18 years of age.
Before using BRYHALI, tell your doctor about all of your medical conditions, including if you:
- have had irritation or other skin reaction to a steroid medicine in the past.
- have a skin infection. You may need medicine to treat the skin infection before using BRYHALI.
- have diabetes.
- have adrenal gland problems.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if BRYHALI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if BRYHALI passes into your breast milk. If you use BRYHALI and breastfeed, do not apply BRYHALI to your nipple or areola to avoid getting BRYHALI into your baby’s mouth.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your doctor if you take other corticosteroids medicines by mouth, or injection, or use other products on your skin that contain corticosteroids.
How should I use BRYHALI?
- Use BRYHALI exactly as your doctor tells you to use it.
- Apply a thin layer of BRYHALI to the affected areas 1 time each day and rub in gently.
- You should not use more than 50 g of BRYHALI in 1 week.
- Do not bandage, wrap, or cover the treated skin area(s) unless your doctor tells you to.
- Avoid using BRYHALI on your face, groin, or underarms (armpits).
- Talk to your doctor if your skin does not improve after 8 weeks of treatment with BRYHALI.
- You should not use BRYHALI longer than 8 weeks unless your doctor tells you to.
- Wash your hands after using BRYHALI unless you are using the medicine to treat your hands.
What are the possible side effects of BRYHALI?
BRYHALI may cause serious side effects, including:
- BRYHALI can pass through your skin. Too much BRYHALI passing through your skin can cause adrenal glands to stop working.
- Cushing’s syndrome, a condition that happens when your body is exposed to too much of the hormone cortisol.
- High blood sugar (hyperglycemia).
- Skin reactions at the treated skin site. Tell your doctor if you get any skin reactions or skin infections.
- Vision problems. BRYHALI may increase your chance of developing cataract(s) and glaucoma. Tell your healthcare provider if you develop blurred vision or other vision problems during treatment with BRYHALI.
- Effects on growth and weight in children.
The most common side effects of BRYHALI include burning, stinging, itching, dryness (application site dermatitis), upper respiratory tract infection and high blood sugar (hyperglycemia).
These are not all the possible side effects of BRYHALI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store BRYHALI?
- Store BRYHALI at room temperature between 68° to 77°F (20° to 25°C).
- Protect from freezing.
- Keep BRYHALI and all medicines out of the reach of children.
General information about the safe and effective use of BRYHALI.
- Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use BRYHALI for a condition for which it was not prescribed. Do not give BRYHALI to other people, even if they have the same condition you have. It may harm them. You can ask your pharmacist or doctor for information about BRYHALI that is written for health professionals.
What are the ingredients in BRYHALI?
Active ingredients: halobetasol propionate
Inactive ingredients: carbomer copolymer type B, carbomer homopolymer type A, diethyl sebacate, edetate disodium dihydrate, light mineral oil, methylparaben, propylparaben, purified water, sodium hydroxide, sorbitan monooleate and sorbitol solution, 70%.
Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by: Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada
Manufactured by: Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada
U.S. Patent Numbers: 8,809,307 and 10,478,502
BRYHALI is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
- For more information, go to
- This Patient Information has been approved by the U.S. Food and Drug Administration.
- 9652104
- Revised: 06/2020
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0187-0002-01
For Topical Use Only
Not for Eye Use
Not for Eye Use
BRYHALI
(halobetasol propionate) Lotion, 0.01%
(halobetasol propionate) Lotion, 0.01%
Rx only
Net Wt. 100 g
Ortho Dermatologics
