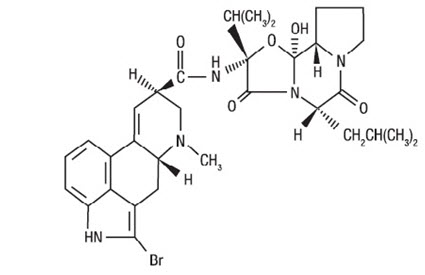
Cycloset
What is Cycloset (Bromocriptine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: A Phase III clinical study evaluating the efficacy and safety of cabergoline tablets versus bromocriptine mesylate tablets in patients with hyperprolactinemia
Summary: The study will enroll 200 women newly diagnosed with peripartum cardiomyopathy within 5 months postpartum in a randomized placebo controlled trial of bromocriptine therapy to evaluate its impact on myocardial recovery and clinical outcomes. Given that bromocriptine prevents breastfeeding, an additional 50 women with peripartum cardiomyopathy excluded from the trial due to a desire to continue brea...
Summary: The study aims to observe changes in dopaminergic genes expression in peripheral tissue upon prolonged dopamine agonist treatment on patients with Restless Legs Syndrome (RLS). Similar studies in Parkinson's disease have shown changes in alpha-synuclein expression, which might offer insights into the dopaminergic gene regulation seen in RLS. The dopamine agonist drugs to be included in this study ...
Related Latest Advances
Brand Information
- Patients with known hypersensitivity to bromocriptine, ergot-related drugs, or any of the excipients in CYCLOSET.
- Patients with syncopal migraine. Bromocriptine increases the likelihood of a hypotensive episode among patients with syncopal migraine. Loss of consciousness during a migraine may reflect dopamine receptor hypersensitivity. CYCLOSET is a dopamine receptor agonist and may, therefore, potentiate the risk for syncope in these patients.
- Postpartum patients. Serious and life-threatening adverse reactions have been reported with bromocriptine use in this population
- Lactating patients. CYCLOSET contains bromocriptine which inhibits lactation
- Hypotension
- Psychotic Disorders
- Somnolence
- Risks in Postpartum Women
- The active ingredient in CYCLOSET (bromocriptine mesylate) is highly bound to serum proteins. Therefore, CYCLOSET may increase the unbound fraction of other concomitantly used highly protein-bound therapies (e.g., salicylates, sulfonamides, chloramphenicol and probenecid), which may alter their effectiveness and risk for side effects.
- CYCLOSET is a dopamine receptor agonist. Concomitant use of dopamine receptor antagonists, such as neuroleptics (e.g., phenothiazines, butyrophenones, thioxanthenes), or metoclopramide may diminish the effectiveness of CYCLOSET, and CYCLOSET may diminish the effectiveness of these other therapies. The concurrent use of CYCLOSET with these agents has not been studied in clinical trials and is not recommended
- CYCLOSET in combination with ergot-related drugs may cause an increase in the occurrence of ergot-related side effects, such as nausea, vomiting, and fatigue, and may also reduce the effectiveness of these ergot therapies when used to treat migraine. The concurrent use of these ergot agents within 6 hours of CYCLOSET dosing is not recommended.
- CYCLOSET is extensively metabolized by the liver via CYP3A4. Therefore, potent inhibitors or inducers of CYP3A4 may increase or reduce the circulating levels of CYCLOSET, respectively. Use caution when co-administering drugs that are inhibitors or inducers of CYP3A4. CYCLOSET dose should not exceed 1.6 mg once daily during concomitant use of a moderate CYP3A4 inhibitor (e.g., erythromycin). Concomitant use of strong CYP3A4 inhibitors (e.g., azole antimycotics, HIV protease inhibitors) with CYCLOSET should be avoided. Ensure adequate washout of the strong CYP3A4 inhibitor drug before initiating CYCLOSET treatment
- There are postmarketing reports of hypertension and tachycardia when bromocriptine was co-administered with sympathomimetic drugs (e.g., phenylpropanolamine and isometheptene) in postpartum women. There are limited clinical trial data supporting the safety of co-administering sympathomimetic drugs and CYCLOSET for more than 10 days. Therefore, concomitant use of these agents with CYCLOSET for more than 10 days duration is not recommended. Also, there are limited clinical trial data supporting the safety of selective 5-hydroxytryptamine