Brand Name
Corlanor
Generic Name
Ivabradine
View Brand Information FDA approval date: April 20, 2015
Classification: Hyperpolarization-activated Cyclic Nucleotide-gated Channel Blocker
Form: Tablet, Solution
What is Corlanor (Ivabradine)?
Ivabradine tablets are hyperpolarization-activated cyclic nucleotide-gated channel blocker indicated: To reduce the risk of hospitalization for worsening heart failure in adult patients with stable, symptomatic chronic heart failure with reduced left ventricular ejection fraction.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Corlanor (ivabradine)
1DOSAGE FORMS AND STRENGTHS
Corlanor Tablets
5 mg: salmon-colored, oval-shaped, film-coated tablet, functionally scored on both edges, debossed with “5” on one face and bisected on the other face. The tablet is scored and can be divided into equal halves to provide a 2.5 mg dose.
7.5 mg: salmon-colored, triangular-shaped, film-coated tablet debossed with “7.5” on one face and plain on the other face.
Corlanor Oral Solution
Corlanor 5 mg/5 mL (1 mg/mL) oral solution is a colorless liquid in an opaque, plastic, ampule containing 5 mg of Corlanor in 5 mL of liquid.
2CONTRAINDICATIONS
Corlanor is contraindicated in patients with:
• Acute decompensated heart failure
• Clinically significant hypotension
• Sick sinus syndrome, sinoatrial block or 3
• Clinically significant bradycardia
• Severe hepatic impairment
• Pacemaker dependence (heart rate maintained exclusively by the pacemaker)
• Concomitant use of strong cytochrome P450 3A4 (CYP3A4) inhibitors
3ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include:
- Atrial Fibrillation
- Bradycardia and Conduction Disturbances
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Patients with Heart Failure
In SHIFT, safety was evaluated in 3,260 patients treated with Corlanor and 3,278 patients given placebo. The median duration of Corlanor exposure was 21.5 months.
The most common adverse drug reactions in the SHIFT trial are shown in Table 2
Luminous Phenomena (Phosphenes)
Phosphenes are phenomena described as a transiently enhanced brightness in a limited area of the visual field, halos, image decomposition (stroboscopic or kaleidoscopic effects), colored bright lights, or multiple images (retinal persistency). Phosphenes are usually triggered by sudden variations in light intensity. Corlanor can cause phosphenes, thought to be mediated through Corlanor’s effects on retinal photoreceptors
Pediatric Patients with Heart Failure
The safety of Corlanor in pediatric patients 6 months to less than 18 years of age is based on a clinical trial
3.2Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or establish a causal relationship to drug exposure.
The following adverse reactions have been identified in adults during post-approval use of Corlanor: syncope, hypotension, torsade de pointes, ventricular fibrillation, ventricular tachycardia, angioedema, erythema, rash, pruritus, urticaria, vertigo, and diplopia, and visual impairment.
4OVERDOSAGE
Overdose may lead to severe and prolonged bradycardia. In the event of bradycardia with poor hemodynamic tolerance, temporary cardiac pacing may be required. Supportive treatment, including intravenous (IV) fluids, atropine, and intravenous beta-stimulating agents such as isoproterenol, may be considered.
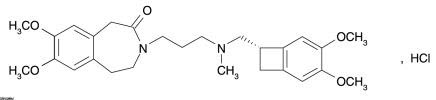
5DESCRIPTION
Corlanor (ivabradine) tablets and oral solution contains ivabradine as the active pharmaceutical ingredient. Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that reduces the spontaneous pacemaker activity of the cardiac sinus node by selectively inhibiting the I
The chemical name for ivabradine hydrochloride is 3-(3-{[((7
Figure 1. Chemical Structure of Ivabradine
Tablets
Corlanor tablets are supplied in 5 mg and 7.5 mg tablets for oral administration. The tablets contain 5 mg and 7.5 mg of ivabradine, as the active ingredient, equivalent to 5.39 mg and 8.09 mg of ivabradine hydrochloride, respectively. The tablets contain the following inactive ingredients: colloidal silicon dioxide, glycerol, hypromellose, lactose monohydrate, magnesium stearate, maize starch, maltodextrin, polyethylene glycol 6000, red iron oxide, titanium dioxide, and yellow iron oxide.
Oral Solution
Corlanor 5 mg/5 mL (1 mg/mL) oral solution is formulated as a sterile, preservative-free, colorless solution for oral administration. Each 5 mL ampule contains 5 mg of ivabradine, equivalent to 5.39 mg ivabradine hydrochloride, as the active ingredient. The solution contains the following inactive ingredients: maltitol and water.
6HOW SUPPLIED/STORAGE AND HANDLING
Tablets:
Corlanor (ivabradine) 5 mg tablets are formulated as salmon-colored, oval-shaped, film-coated tablets functionally scored on both edges, marked with “5” on one face and bisected on the other face. They are supplied as follows:
- Bottles of 60 tablets with child-resistant closure (NDC 55513-800-60)
Corlanor (ivabradine) 7.5 mg tablets are formulated as salmon-colored, triangular-shaped, film-coated tablets debossed with “7.5” on one face and plain on the other face. They are supplied as follows:
- Bottles of 60 tablets with child-resistant closure (NDC 55513-810-60)
Oral Solution:
Corlanor (ivabradine) oral solution is a colorless liquid supplied in an opaque, low density polyethylene (LDPE) plastic ampule. Each 5 mL ampule is individually packaged in a child-resistant foil pouch and supplied in cartons containing 28 foil pouches. Corlanor oral solution is supplied as 5 mg/5 mL (1 mg/mL) (NDC 55513-813-01).
Storage
Store Corlanor tablets and oral solution at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F - 86°F)
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling
- Fetal Toxicity
Advise pregnant women of the potential risks to a fetus.
Advise females of reproductive potential to use effective contraception and to notify their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1), (8.3)]. - Low Heart Rate
Advise patients to report significant decreases in heart rate or symptoms such as dizziness, fatigue, or hypotension [see Warnings and Precautions (5.3)]. - Atrial Fibrillation
Advise patients to report symptoms of atrial fibrillation, such as heart palpitations or racing, chest pressure, or worsened shortness of breath [see Warnings and Precautions (5.2)]. - Phosphenes
Advise patients about the possible occurrence of luminous phenomena (phosphenes). Advise patients to use caution if they are driving or using machines in situations where sudden changes in light intensity may occur, especially when driving at night. Advise patients that phosphenes may subside spontaneously during continued treatment with Corlanor [see Adverse Reactions (6.1)]. - Drug Interactions
Advise patients to avoid ingestion of grapefruit juice and St. John’s wort [see Drug Interactions(7.1)]. - Intake with Food
Advise patients to take Corlanor twice daily with food [see Dosage and Administration (2)]. - Oral Solution
Advise parents/caregivers on preparation and administration instructions including the use of a calibrated oral syringe and a medicine cup (provided by the pharmacy) to avoid dosing errors [see Dosage and Administration (2.2)].
Advise parents/caregivers that the oral solution should not be administered by the child.
Advise parents/caregivers to not double up doses (e.g., if patient spits out the drug or caregiver forgets to give the drug at the prescribed time).
Advise parents/caregivers to throw away the unused product remaining in the cup immediately after drawing up the prescribed dose in the syringe.
Corlanor
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
Patent:
© 2015, 2017, 2019, 2021 Amgen Inc. All rights reserved.
8PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
AMGEN
NDC 55513-800-60
5
Corlanor
Each tablet contains 5 mg ivabradine equivalent
Rx Only
60

9PRINCIPAL DISPLAY PANEL - 7.5 mg Tablet Bottle Label
AMGEN
NDC 55513-810-60
7.5
Corlanor
Each tablet contains 7.5 mg ivabradine equivalent
Rx Only
60

10PRINCIPAL DISPLAY PANEL - 5 mg/5 mL Ampule Carton
NDC 55513-813-28
Caregiver/Patient: Read the included "Instructions for Use" insert to be sure you prepare and administer the correct dose.
AMGEN
ATTENTION
Corlanor
5 mg/5 mL (1 mg/mL)
Sterile Oral Solution in an Ampule
Rx Only
Contains 28
