Generic Name
Adalimumab-ADAZ
Brand Names
Amjevita, Hadlima, Adalimumab-bwwd, Adalimumab, Yusimry, Hyrimoz
FDA approval date: January 31, 2023
Classification: Tumor Necrosis Factor Blocker
Form: Injection, Kit, Solution
What is Amjevita (Adalimumab-ADAZ)?
Plaque Psoriasis HYRIMOZ is indicated for the treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy, and when other systemic therapies are medically less appropriate. HYRIMOZ should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician. Ulcerative Colitis HYRIMOZ is indicated for the treatment of moderately to severely active ulcerative colitis in adult patients. Limitations of Use: The effectiveness of adalimumab products has not been established in patients who have lost response to or were intolerant to TNF-blockers [see Clinical Studies (1. HYRIMOZ is a tumor necrosis factor -blocker indicated for: Rheumatoid Arthritis .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
AMJEVITA (adalimumab-atto)
1DOSAGE FORMS AND STRENGTHS
AMJEVITA is a clear, colorless to slightly yellow solution available as:
- Prefilled SureClick Autoinjector
- Injection: 80 mg/0.8 mL in a single-dose prefilled SureClick autoinjector.
- Injection: 40 mg/0.8 mL in a single-dose prefilled SureClick autoinjector.
- Injection: 40 mg/0.4 mL in a single-dose prefilled SureClick autoinjector.
- Prefilled Syringe
- Injection: 80 mg/0.8 mL in a single-dose prefilled glass syringe.
- Injection: 40 mg/0.8 mL in a single-dose prefilled glass syringe.
- Injection: 40 mg/0.4 mL in a single-dose prefilled glass syringe.
- Injection: 20 mg/0.4 mL in a single-dose prefilled glass syringe.
- Injection: 20 mg/0.2 mL in a single-dose prefilled glass syringe.
- Injection: 10 mg/0.2 mL in a single-dose prefilled glass syringe.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Infections
- Malignancies
- Hypersensitivity Reactions
- Hepatitis B Virus Reactivation
- Neurologic Reactions
- Hematological Reactions
- Heart Failure
- Autoimmunity
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reaction with adalimumab was injection site reactions. In placebo-controlled trials, 20% of subjects treated with adalimumab developed injection site reactions (erythema and/or itching, hemorrhage, pain or swelling), compared to 14% of subjects receiving placebo. Most injection site reactions were described as mild and generally did not necessitate drug discontinuation.
The proportion of subjects who discontinued treatment due to adverse reactions during the double-blind, placebo-controlled portion of studies in subjects with RA (i.e., Studies RA-I, RA-II, RA-III and RA-IV) was 7% for subjects taking adalimumab and 4% for placebo-treated subjects. The most common adverse reactions leading to discontinuation of adalimumab in these RA studies were clinical flare reaction (0.7%), rash (0.3%) and pneumonia (0.3%).
3.2Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of adalimumab or of other adalimumab products.
There are two assays that have been used to measure anti-adalimumab antibodies. With the ELISA, antibodies to adalimumab could be detected only when serum adalimumab concentrations were < 2 mcg/mL. The ECL assay can detect anti-adalimumab antibody titers independent of adalimumab concentrations in the serum samples. The incidence of anti-adalimumab antibody (AAA) development in patients treated with adalimumab are presented in Table 2.
3.3Postmarketing Experience
The following adverse reactions have been identified during post-approval use of adalimumab products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to adalimumab products exposure.
Gastrointestinal disorders: Diverticulitis, large bowel perforations including perforations associated with diverticulitis and appendiceal perforations associated with appendicitis, pancreatitis
General disorders and administration site conditions: Pyrexia
Hepato-biliary disorders: Liver failure, hepatitis, autoimmune hepatitis
Immune system disorders: Sarcoidosis
Neoplasms benign, malignant and unspecified (including cysts and polyps): Merkel Cell Carcinoma (neuroendocrine carcinoma of the skin)
Nervous system disorders: Demyelinating disorders (e.g., optic neuritis, Guillain-Barré syndrome), cerebrovascular accident
Respiratory disorders: Interstitial lung disease, including pulmonary fibrosis, pulmonary embolism
Skin reactions: Stevens Johnson Syndrome, cutaneous vasculitis, erythema multiforme, new or worsening psoriasis (all sub-types including pustular and palmoplantar), alopecia, lichenoid skin reaction
Vascular disorders: Systemic vasculitis, deep vein thrombosis
4OVERDOSAGE
Doses up to 10 mg/kg have been administered to patients in clinical trials without evidence of dose-limiting toxicities. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment instituted immediately.
Consider contacting the Poison Help line (1-800-222-1222) or medical toxicologist for additional overdose management recommendations.
5DESCRIPTION
Adalimumab-atto is a tumor necrosis factor blocker. Adalimumab-atto is a recombinant human IgG1 monoclonal antibody with human derived heavy and light chain variable regions and human IgG1:k constant regions. Adalimumab-atto is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary (CHO)) expression system and is purified by a process that includes specific viral inactivation and removal steps. It consists of 1330 amino acids and has a molecular weight of approximately 148 kilodaltons.
AMJEVITA
Each 80 mg/0.8 mL prefilled syringe or prefilled autoinjector delivers 0.8 mL (80 mg) of drug product. Each 0.8 mL of AMJEVITA is formulated with L-lactic acid (1.7 mg), polysorbate 80 (0.8 mg), sodium hydroxide for pH adjustment, sucrose (67 mg), and Water for Injection, USP, pH 5.2.
Each 40 mg/0.8 mL prefilled syringe or prefilled autoinjector delivers 0.8 mL (40 mg) of drug product. Each 0.8 mL of AMJEVITA is formulated with glacial acetic acid (0.48 mg), polysorbate 80 (0.8 mg), sodium hydroxide for pH adjustment, sucrose (72 mg), and Water for Injection, USP, pH 5.2.
Each 40 mg/0.4 mL prefilled syringe or prefilled autoinjector delivers 0.4 mL (40 mg) of drug product. Each 0.4 mL of AMJEVITA is formulated with L-lactic acid (0.9 mg), polysorbate 80 (0.4 mg), sodium hydroxide for pH adjustment, sucrose (34 mg), and Water for Injection, USP, pH 5.2.
Each 20 mg/0.4 mL prefilled syringe delivers 0.4 mL (20 mg) of drug product. Each 0.4 mL of AMJEVITA is formulated with glacial acetic acid (0.24 mg), polysorbate 80 (0.4 mg), sodium hydroxide for pH adjustment, sucrose (36 mg), and Water for Injection, USP, pH 5.2.
Each 20 mg/0.2 mL prefilled syringe delivers 0.2 mL (20 mg) of drug product. Each 0.2 mL of AMJEVITA is formulated with L-lactic acid (0.4 mg), polysorbate 80 (0.2 mg), sodium hydroxide for pH adjustment, sucrose (17 mg), and Water for Injection, USP, pH 5.2.
Each 10 mg/0.2 mL prefilled syringe delivers 0.2 mL (10 mg) of drug product. Each 0.2 mL of AMJEVITA is formulated with glacial acetic acid (0.12 mg), polysorbate 80 (0.2 mg), sodium hydroxide for pH adjustment, sucrose (18 mg), and Water for Injection, USP, pH 5.2.
6REFERENCES
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 17 Registries, 2000-2007.
7HOW SUPPLIED/STORAGE AND HANDLING
AMJEVITA
The following packaging configurations are available.
8PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
9INSTRUCTIONS FOR USE
AMJEVITA
This Instructions for Use contains information on how to inject AMJEVITA with a SureClick autoinjector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of AMJEVITA at home, you should receive training on the right way to prepare and inject AMJEVITA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the AMJEVITA autoinjector is for injection under the skin (subcutaneous injection). See the AMJEVITA Medication Guide for information about AMJEVITA.
1. Important Information You Need to Know Before Injecting AMJEVITA
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the autoinjector if the carton is damaged or the seal is broken.
- Do not use the autoinjector after the expiration date on the label.
- Do not shake the autoinjector.
- Do not remove the yellow cap from the autoinjector until you are ready to inject.
- Do not use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
- The autoinjector is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit www.amjevita.com.
For additional information and answers to frequently asked questions, visit www.amjevita.com.
Where to get help:
If you want more information or help using AMJEVITA:
If you want more information or help using AMJEVITA:
- Contact your healthcare provider,
- Visit
- Call 1-800-77-AMGEN (1-800-772-6436)
2. Storing and Preparing to Inject AMJEVITA
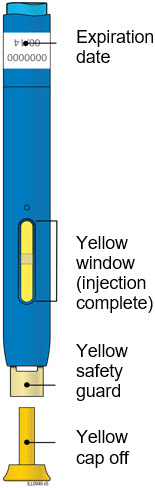
2a Refrigerate the autoinjector carton until you are ready to use it.
- Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
2b Wait 15 to 30 minutes for the autoinjector to reach room temperature.
- Remove the number of autoinjectors you need for your injection and put any unused autoinjectors back into the refrigerator.
- Let the autoinjector warm up naturally.
- Do not heat the autoinjector with hot water, a microwave, or direct sunlight.
- Do not shake the autoinjector at any time.
- Using the autoinjector at room temperature makes sure the full dose is delivered and allows for a more comfortable injection.
2c You may keep AMJEVITA at room temperature for up to
- For example, when you are traveling, you may keep AMJEVITA at room temperature.
2d Inspect the medicine. It should be clear and colorless to pale yellow.
- It is okay to see air bubbles in the autoinjector.
- Do not use AMJEVITA if the medicine is cloudy, discolored, or has flakes or particles.
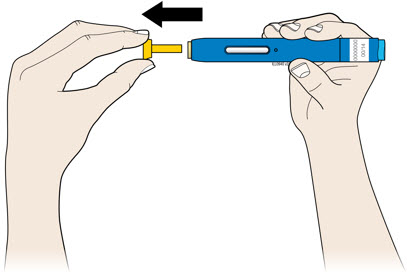
2e Check the expiration date (Exp.) and inspect the autoinjector for damage.
- Do not use the autoinjector if the expiration date has passed.
- Do not use the autoinjector if:
- the yellow cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
3. Getting Ready for Your Injection
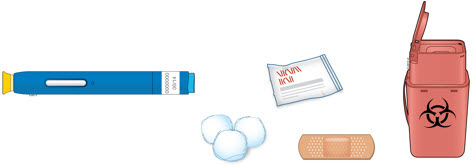
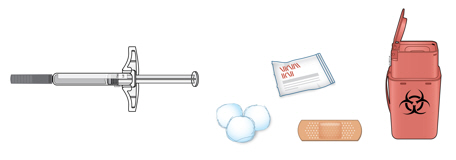
3a Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- AMJEVITA autoinjector (room temperature)
- Sharps disposal container [see Disposing of AMJEVITA and Checking the Injection Site]
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad

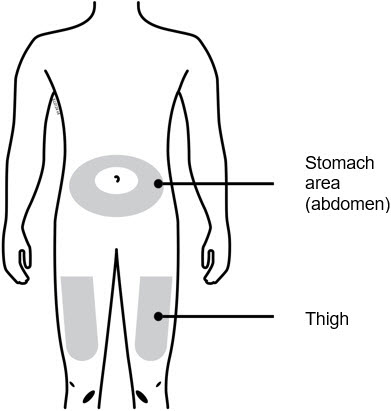
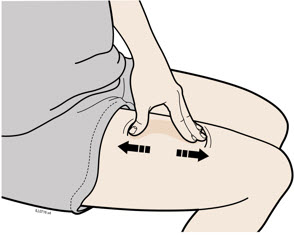
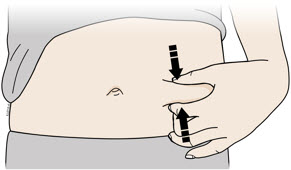
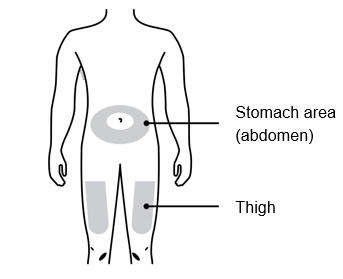
3b Inject into 1 of these locations.
- Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Choose a different site for each injection.
3c Wash hands thoroughly with soap and water.
3d Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting AMJEVITA
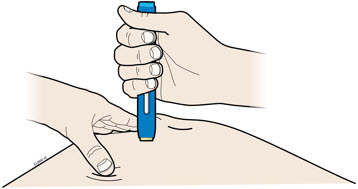
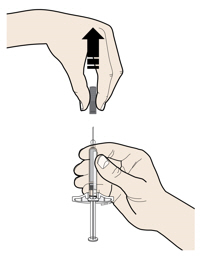
4a Grasp the autoinjector so you can see the window. Pull the yellow cap straight off. You may need to pull hard.
- Do not twist, bend or wiggle the yellow cap to pull it off.
- Never put the yellow cap back on. It may damage the needle.
- Do not put your finger inside the cream safety guard.
- It is normal to see a drop of medicine at the end of the needle or cream safety guard.
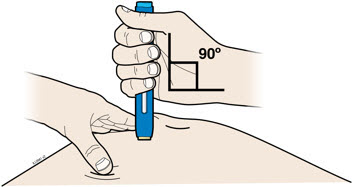
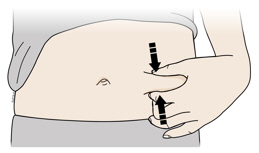
4b Pinch the skin to create a firm surface at the injection site. Place the cream safety guard straight against the pinched skin.
- Keep the skin
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
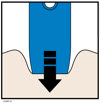
4c Firmly push the autoinjector down until the cream safety guard stops moving. Hold the autoinjector down, do not lift.
- The cream safety guard pushes in and unlocks the light blue start button.
4d Keep pushing the autoinjector down and press the light blue start button to start the injection.
- You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the light blue start button.
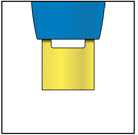
4e Keep pushing the autoinjector down. When the window is fully yellow, the injection is complete.
- The injection may take up to
- You may hear or feel a click.
- Lift the autoinjector away from your skin.
- The cream safety guard locks around the needle.
5. Disposing of AMJEVITA and Checking the Injection Site
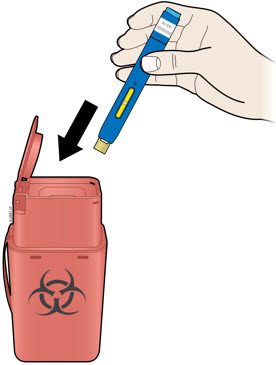
5a Place the used autoinjector and yellow cap in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the autoinjector.
- Do not touch the cream safety guard.
5b Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMJEVITA (adalimumab-atto)
Manufactured by:
©2024 Amgen Inc. All rights reserved.
10INSTRUCTIONS FOR USE
AMJEVITA
This Instructions for Use contains information on how to inject AMJEVITA with a prefilled syringe.
If your healthcare provider decides that you or a caregiver may be able to give your injections of AMJEVITA at home, you should receive training on the right way to prepare and inject AMJEVITA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the AMJEVITA prefilled syringe is for injection under the skin (subcutaneous injection). See the AMJEVITA Medication Guide for information about AMJEVITA.
1. Important Information You Need to Know Before Injecting AMJEVITA
Dosing:
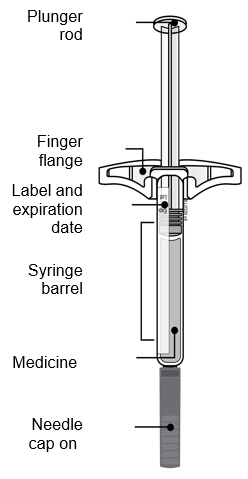
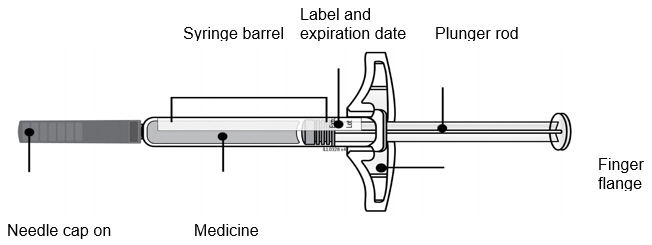
- AMJEVITA comes in 2 different doses: 20 mg/0.4 mL and 40 mg/0.8 mL. Check your prescription to make sure you have the correct dose.
- The color and look of the prefilled syringe will be different for each dose. The amount of medicine in the syringe will also be different for each dose.
- For example, it is okay for the 20 mg/0.4 mL dose to have a small amount of medicine and the 40 mg/0.8 mL to have a large amount of medicine. Check the illustrations below to see what your dose looks like in the syringe.
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the syringe if the carton is damaged or the seal is broken.
- Do not use the syringe after the expiration date on the label.
- Do not shake the syringe.
- Do not remove the needle cap from the syringe until you are ready to inject.
- Do not use the syringe if it has been frozen.
- Do not use the syringe if it has been dropped on a hard surface. Part of the syringe may be broken even if you cannot see the break. Use a new syringe, and call 1-800-77-AMGEN (1-800-772-6436).
- The syringe is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit
Where to get help:
If you want more information or help using AMJEVITA:
- Contact your healthcare provider,
- Visit
- Call 1-800-77-AMGEN (1-800-772-6436)
2. Storing and Preparing to Inject AMJEVITA
2a Refrigerate the syringe carton until you are ready to use it.
- Keep the syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the syringe in the original carton to protect it from light or physical damage.
- Do not freeze the syringe.
- Do not store the syringe in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
2b Grasp the syringe by the body and remove it from the carton.
- Do not grab the finger grip, plunger rod, or the needle cap.
- Remove the number of syringes you need for your injection.
- Put any unused syringes back into the refrigerator.
2c Wait 15 to 30 minutes for the syringe to reach room temperature.
- Let the syringe warm up naturally.
- Do not heat the syringe with hot water, a microwave, or direct sunlight.
- Do not shake the syringe at any time.
- Using the syringe at room temperature allows for a more comfortable injection
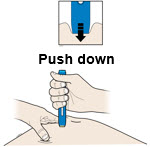
2d You may keep AMJEVITA at room temperature for up to 14 days, if needed.
- For example, when you are traveling, you may keep AMJEVITA at room temperature.
2e Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- AMJEVITA syringe (room temperature)
- Sharps disposal container [see Disposing of AMJEVITA and Checking the Injection Site]
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad
3. Getting Ready for Your Injection
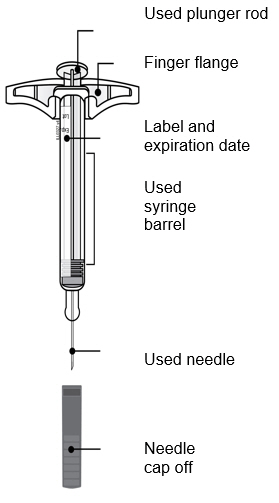
3a Inspect the medicine. It should be clear and colorless to pale yellow.
- It is okay to see air bubbles in the syringe.
- Do not use AMJEVITA if the medicine is cloudy, discolored, or has flakes or particles.
3b Check the expiration date (Exp.) and inspect the syringe for damage.
- Do not use the syringe if the expiration date has passed.
- Do not use the syringe if:
- the needle cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
3c Inject into 1 of these locations.
- Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Choose a different site for each injection.
3d Wash hands thoroughly with soap and water.
3e Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting AMJEVITA
4a Pull the needle cap straight off while holding the syringe body.
- Do not twist or bend the needle cap.
- Never put the needle cap back on. It may damage the needle.
- Do not let anything touch the needle after you remove the needle cap.
- Do not place the uncapped syringe on any surface after you remove the needle cap.
- Do not try to push air bubbles out of the syringe. It is okay to see air bubbles.
- It is normal to see a drop of medicine come out of the needle.
4b Place the needle cap in the sharps disposal container.
4c Pinch the skin around the injection site before injection.
- Pinch the skin between the thumb and pointer (index) finger to create a bump for the injection.
- If possible, the bump should be about 2 inches wide.
4d Insert the needle into the pinched skin at a 45-degree angle.
- Do not place your finger on the plunger rod while inserting the needle, as this may result in lost medicine.
4e Slowly press the plunger rod all the way down until it reaches the bottom of the syringe to inject the medicine.
- Do not pull back on the plunger rod at any time.
- Do not remove the syringe until all of the medicine has been injected.
5. Disposing of AMJEVITA and Checking the Injection Site
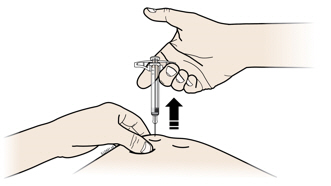
5a Place the used syringe in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the syringe.
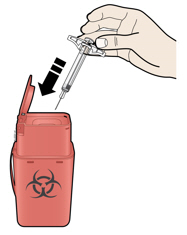
5b Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMJEVITA (adalimumab-atto)
Manufactured by:
©2024 Amgen Inc. All rights reserved.
11INSTRUCTIONS FOR USE
AMJEVITA
This Instructions for Use contains information on how to inject AMJEVITA with a prefilled syringe.
If your healthcare provider decides that a caregiver may be able to give your injections of AMJEVITA at home, they should receive training on the right way to prepare and inject AMJEVITA. Do not try to inject until they have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the AMJEVITA prefilled syringe is for injection under the skin (subcutaneous injection). See the AMJEVITA
1. Important Information You Need to Know Before Injecting AMJEVITA
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the syringe if the carton is damaged or the seal is broken.
- Do not use the syringe after the expiration date on the label.
- Do not shake the syringe.
- Do not remove the needle cap from the syringe until you are ready to inject.
- Do not use the syringe if it has been frozen.
- Do not use the syringe if it has been dropped on a hard surface. Part of the syringe may be broken even if you cannot see the break. Use a new syringe, and call 1-800-77-AMGEN (1-800-772-6436).
- The syringe is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit
Where to get help:
If you want more information or help using AMJEVITA:
- Contact your healthcare provider,
- Visit www.amjevita.com, or
- Call 1-800-77-AMGEN (1-800-772-6436)
2. Storing and Preparing to Inject AMJEVITA
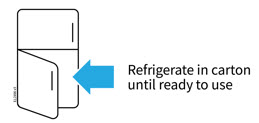
2a Refrigerate the syringe carton until you are ready to use it.
- Keep the syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the syringe in the original carton to protect it from light or physical damage.
- Do not freeze the syringe.
- Do not store the syringe in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
Important: Keep the syringe out of the sight and reach of children.

2b Grasp the syringe by the body and remove it from the carton.
- Do not grab the finger grip, plunger rod, or the needle cap.
- Remove the number of syringes you need for your injection.
- Put any unused syringes back into the refrigerator.
2c Wait 15 to 30 minutes for the syringe to reach room temperature.
- Let the syringe warm up naturally.
- Do not heat the syringe with hot water, a microwave or direct sunlight.
- Do not shake the syringe at any time.
- Using the syringe at room temperature allows for a more comfortable injection

2d You may keep AMJEVITA at room temperature for up to 14 days, if needed.
- For example when you are traveling, you may keep AMJEVITA at room temperature.
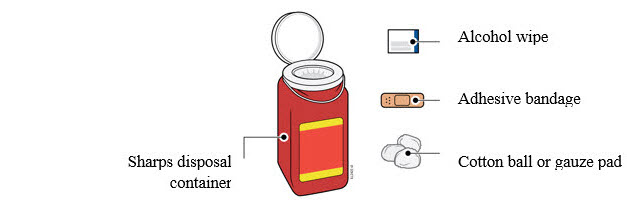
2e Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- AMJEVITA syringe (room temperature)
- Sharps disposal container [see
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad
3. Getting Ready for Your Injection
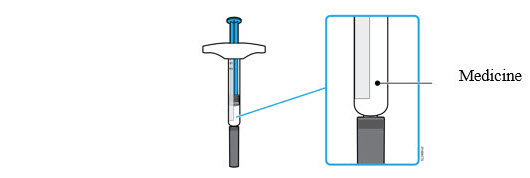
3a Inspect the medicine. It should be clear and colorless to pale yellow.
- It is okay to see air bubbles in the syringe.
- Do not use AMJEVITA if the medicine is cloudy, discolored or has flakes.
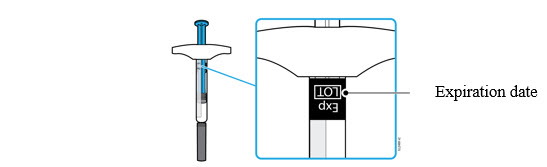
3b Check the expiration date (Exp.) and inspect the syringe for damage.
- Do not use the syringe if the expiration date has passed.
- Do not use the syringe if:
- the needle cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
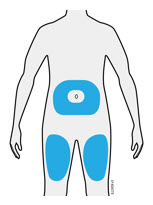
3c Inject into 1 of these locations.
- Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Choose a different site for each injection.
3d Wash hands thoroughly with soap and water.
3e Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting AMJEVITA
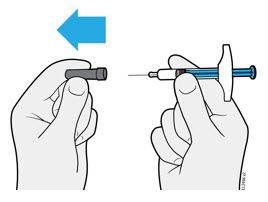
4a Pull the needle cap straight off while holding the syringe body.
- Do not twist or bend the needle cap.
- Never put the needle cap back on. It may damage the needle.
- Do not let anything touch the needle after you remove the needle cap.
- Do not place the uncapped syringe on any surface after you remove the needle cap.
- Do not try to push air bubbles out of the syringe. It is okay to see air bubbles.
- It is normal to see a drop of medicine come out of the needle.
4b Place the needle cap in the sharps disposal container.
4c Pinch the skin around the injection site before injection.
- Pinch the skin between the thumb and pointer (index) finger to create a bump for injection.
- If possible, the bump should be about 2 inches wide.
4d Insert the needle into the pinched skin at about a 45-degree angle.
- Do not place your finger on the plunger rod while inserting the needle, as this may result in lost medicine.
4e Slowly press the plunger rod all the way down until it reaches the bottom to inject the medicine.
- Do not pull back on the plunger rod at any time.
- Do not remove the syringe until all of the medicine has been injected.
5. Disposing of AMJEVITA and Checking the Injection Site
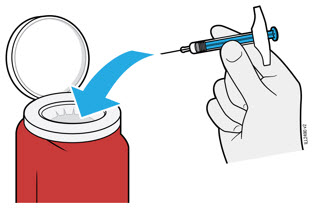
5a Place the used syringe in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the syringe.
5b Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on the injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container you may use a household container that is:
Disposing of sharps containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
This Instructions for Use has been approved by the US Food and Drug Administration.
AMJEVITA
Manufactured by:
12INSTRUCTIONS FOR USE
AMJEVITA
This Instructions for Use contains information on how to inject AMJEVITA with a SureClick autoinjector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of AMJEVITA at home, you should receive training on the right way to prepare and inject AMJEVITA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the AMJEVITA autoinjector is for injection under the skin (subcutaneous injection). See the AMJEVITA
1. Important Information You Need to Know Before Injecting AMJEVITA
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the autoinjector if the carton is damaged or the seal is broken.
- Do not use the autoinjector after the expiration date on the label.
- Do not shake the autoinjector.
- Do not remove the yellow cap from the autoinjector until you are ready to inject.
- Do not use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
- The autoinjector is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit
Where to get help:
If you want more information or help using AMJEVITA:
- Contact your healthcare provider,
- Visit
- Call 1-800-77-AMGEN (1-800-772-6436)
2. Storing and Preparing to Inject AMJEVITA
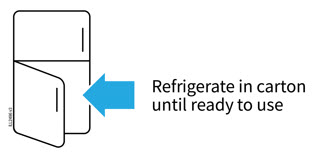
2a Refrigerate the autoinjector carton until you are ready to use it.
- Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
2b Wait 15 to 30 minutes for the autoinjector to reach room temperature.
- Remove the number of autoinjectors you need for your injection and put any unused autoinjectors back into the refrigerator.
- Let the autoinjector warm up naturally.
- Do not heat the autoinjector with hot water, a microwave, or direct sunlight.
- Do not shake the autoinjector at any time.
- Using the autoinjector at room temperature makes sure the full dose is delivered and allows for a more comfortable injection.

2c You may keep AMJEVITA at room temperature for up to 14 days, if needed.
- For example, when you are traveling, you may keep AMJEVITA at room temperature.
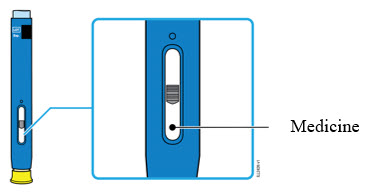
2d Inspect the medicine. It should be clear and colorless to pale yellow.
- It is okay to see air bubbles in the autoinjector.
- Do not use AMJEVITA if the medicine is cloudy, discolored, or has flakes or particles.
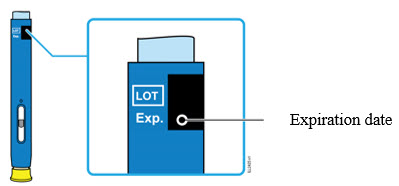
2e Check the expiration date (Exp.) and inspect the autoinjector for damage.
- Do not use the autoinjector if the expiration date has passed.
- Do not use the autoinjector if:
- the yellow cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
3. Getting Ready for Your Injection
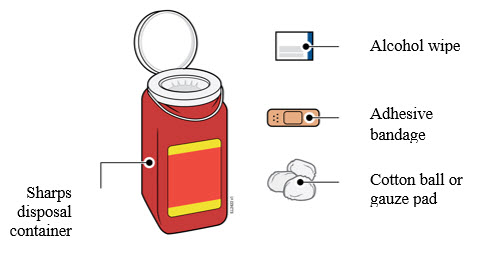
3a Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- AMJEVITA autoinjector (room temperature)
- Sharps disposal container [see
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad
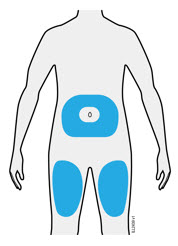
3b Inject into 1 of these locations.
- Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Choose a different site for each injection.
3c Wash hands thoroughly with soap and water.
3d Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting AMJEVITA
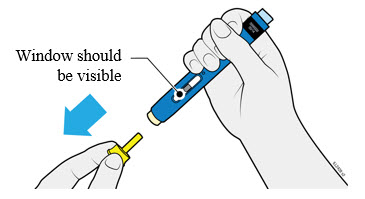
4a Grasp the autoinjector so you can see the window. Pull the yellow cap straight off. You may need to pull hard.
- Do not twist, bend or wiggle the yellow cap to pull it off.
- Never put the yellow cap back on. It may damage the needle.
- Do not put your finger inside the cream safety guard.
- It is normal to see a drop of medicine at the end of the needle or cream safety guard.
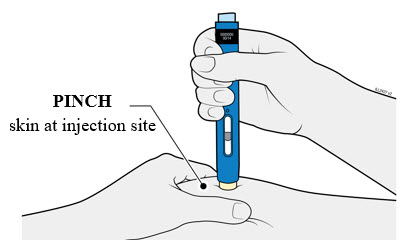
4b Pinch the skin to create a firm surface at the injection site. Place the cream safety guard straight against the pinched skin.
- Keep the skin
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
4c Firmly push the autoinjector down until the cream safety guard stops moving. Hold the autoinjector down, do not lift.
- The cream safety guard pushes in and unlocks the light blue start button.
4d Keep pushing the autoinjector down and press the light blue start button to start the injection.
- You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the light blue start button.
4e Keep pushing the autoinjector down. When the window is fully yellow, the injection is complete.
- The injection may take up to
- You may hear or feel a click.
- Lift the autoinjector away from your skin.
- The cream safety guard locks around the needle.
5. Disposing of AMJEVITA and Checking the Injection Site
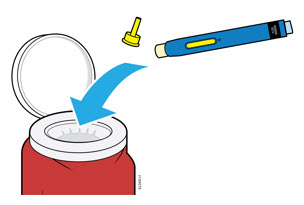
5a Place the used autoinjector and yellow cap in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the autoinjector.
- Do not touch the cream safety guard.
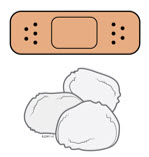
5b Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMJEVITA
Manufactured by:
13INSTRUCTIONS FOR USE
AMJEVITA
This Instructions for Use contains information on how to inject AMJEVITA with a SureClick autoinjector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of AMJEVITA at home, you should receive training on the right way to prepare and inject AMJEVITA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the AMJEVITA autoinjector is for injection under the skin (subcutaneous injection). See the AMJEVITA
1. Important Information You Need to Know Before Injecting AMJEVITA
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the autoinjector if the carton is damaged or the seal is broken.
- Do not use the autoinjector after the expiration date on the label.
- Do not shake the autoinjector.
- Do not remove the yellow cap from the autoinjector until you are ready to inject.
- Do not use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
- The autoinjector is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit
Where to get help:
If you want more information or help using AMJEVITA:
- Contact your healthcare provider,
- Visit
- Call 1-800-77-AMGEN (1-800-772-6436)
2. Storing and Preparing to Inject AMJEVITA
2a Refrigerate the autoinjector carton until you are ready to use it.
- Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
2b Wait 15 to 30 minutes for the autoinjector to reach room temperature.
- Remove the number of autoinjectors you need for your injection and put any unused autoinjectors back into the refrigerator.
- Let the autoinjector warm up naturally.
- Do not heat the autoinjector with hot water, a microwave, or direct sunlight.
- Do not shake the autoinjector at any time.
- Using the autoinjector at room temperature makes sure the full dose is delivered and allows for a more comfortable injection.

2c You may keep AMJEVITA at room temperature for up to 14 days, if needed.
- For example, when you are traveling, you may keep AMJEVITA at room temperature.

2d Inspect the medicine. It should be clear and colorless to pale yellow.
- It is okay to see air bubbles in the autoinjector.
- Do not use AMJEVITA if the medicine is cloudy, discolored, or has flakes or particles.
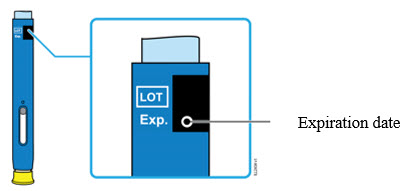
2e Check the expiration date (Exp.) and inspect the autoinjector for damage.
- Do not use the autoinjector if the expiration date has passed.
- Do not use the autoinjector if:
- the yellow cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
3. Getting Ready for Your Injection
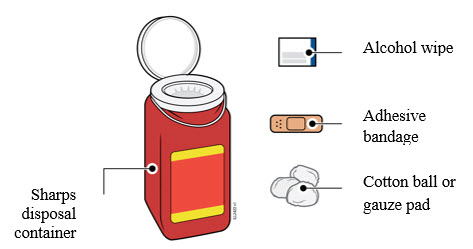
3a Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- AMJEVITA autoinjector (room temperature)
- Sharps disposal container [see
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad
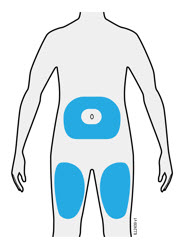
3b Inject into 1 of these locations.
- Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Choose a different site for each injection.
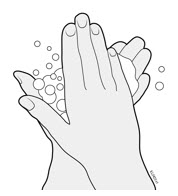
3c Wash hands thoroughly with soap and water.
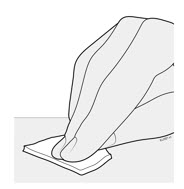
3d Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting AMJEVITA
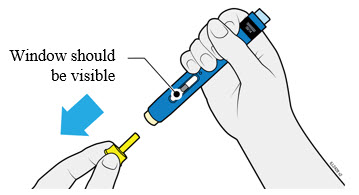
4a Grasp the autoinjector so you can see the window. Pull the yellow cap straight off. You may need to pull hard.
- Do not twist, bend or wiggle the yellow cap to pull it off.
- Never put the yellow cap back on. It may damage the needle.
- Do not put your finger inside the cream safety guard.
- It is normal to see a drop of medicine at the end of the needle or cream safety guard.
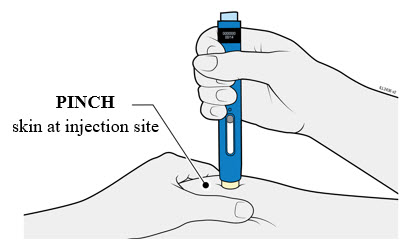
4b Pinch the skin to create a firm surface at the injection site. Place the cream safety guard straight against the pinched skin.
- Keep the skin
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
4c Firmly push the autoinjector down until the cream safety guard stops moving. Hold the autoinjector down, do not lift.
- The cream safety guard pushes in and unlocks the light blue start button.
4d Keep pushing the autoinjector down and press the light blue start button to start the injection.
- You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the light blue start button.
4e Keep pushing the autoinjector down. When the window is fully yellow, the injection is complete.
- The injection may take up to
- You may hear or feel a click.
- Lift the autoinjector away from your skin.
- The cream safety guard locks around the needle.
5. Disposing of AMJEVITA and Checking the Injection Site
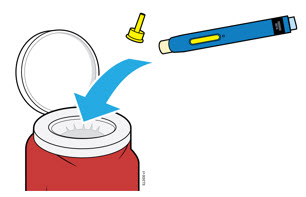
5a Place the used autoinjector and yellow cap in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the autoinjector.
- Do not touch the cream safety guard.
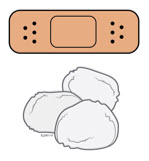
5b Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMJEVITA
Manufactured by:
14INSTRUCTIONS FOR USE
AMJEVITA
This Instructions for Use contains information on how to inject AMJEVITA with a prefilled syringe.
If your healthcare provider decides that you or a caregiver may be able to give your injections of AMJEVITA at home, you should receive training on the right way to prepare and inject AMJEVITA. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the AMJEVITA prefilled syringe is for injection under the skin (subcutaneous injection). See the AMJEVITA
1. Important Information You Need to Know Before Injecting AMJEVITA
Dosing:
- AMJEVITA comes in 3 different doses: 20 mg/0.2 mL, 40 mg/0.4 mL, and 80 mg/0.8 mL. Check your prescription to make sure you have the correct dose.
- The color and look of the prefilled syringe will be different for each dose. The amount of medicine in the syringe will also be different for each dose.
- For example, it is okay for the 20 mg/0.2 mL dose to have a small amount of medicine and the 80 mg/0.8 mL to have a large amount of medicine. Check the illustrations below to see what your dose looks like in the syringe.
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Do not use the syringe if the carton is damaged or the seal is broken.
- Do not use the syringe after the expiration date on the label.
- Do not shake the syringe.
- Do not remove the needle cap from the syringe until you are ready to inject.
- Do not use the syringe if it has been frozen.
- Do not use the syringe if it has been dropped on a hard surface. Part of the syringe may be broken even if you cannot see the break. Use a new syringe, and call 1-800-77-AMGEN (1-800-772-6436).
- The syringe is not made with natural rubber latex.
Frequently asked questions:
For additional information and answers to frequently asked questions, visit
Where to get help:
If you want more information or help using AMJEVITA:
- Contact your healthcare provider,
- Visit
- Call 1-800-77-AMGEN (1-800-772-6436)
2. Storing and Preparing to Inject AMJEVITA
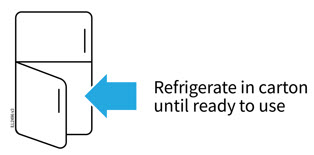
2a Refrigerate the syringe carton until you are ready to use it.
- Keep the syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the syringe in the original carton to protect it from light or physical damage.
- Do not freeze the syringe.
- Do not store the syringe in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
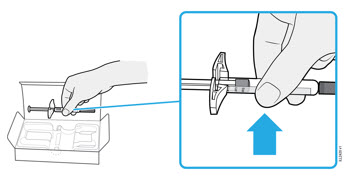
2b Grasp the syringe by the body and remove it from the carton.
- Do not grab the finger grip, plunger rod, or the needle cap.
- Remove the number of syringes you need for your injection.
- Put any unused syringes back into the refrigerator.
2c Wait 15 to 30 minutes for the syringe to reach room temperature.
- Let the syringe warm up naturally.
- Do not heat the syringe with hot water, a microwave, or direct sunlight.
- Do not shake the syringe at any time.
- Using the syringe at room temperature allows for a more comfortable injection

2d You may keep AMJEVITA at room temperature for up to 14 days, if needed.
- For example, when you are traveling, you may keep AMJEVITA at room temperature.
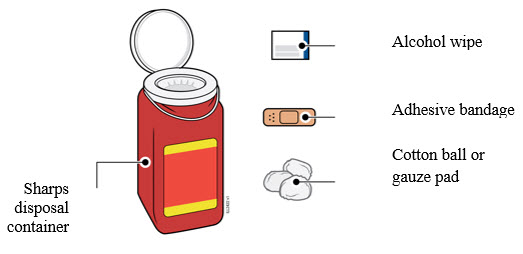
2e Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- AMJEVITA syringe (room temperature)
- Sharps disposal container [see
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad
3. Getting Ready for Your Injection
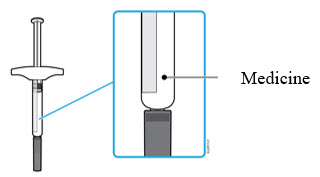
3a Inspect the medicine. It should be clear and colorless to pale yellow.
- It is okay to see air bubbles in the syringe.
- Do not use AMJEVITA if the medicine is cloudy, discolored, or has flakes or particles.
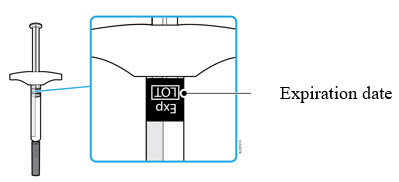
3b Check the expiration date (Exp.) and inspect the syringe for damage.
- Do not use the syringe if the expiration date has passed.
- Do not use the syringe if:
- the needle cap is missing or loose.
- it has cracks or broken parts.
- it has been dropped on a hard surface.
- Make sure you have the right medicine and dose.
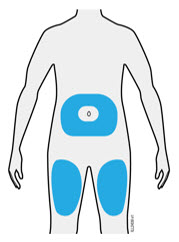
3c Inject into 1 of these locations.
- Inject into the front of your thigh or stomach (except for 2 inches around your belly button).
- Choose a different site for each injection.
3d Wash hands thoroughly with soap and water.
3e Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
4. Injecting AMJEVITA
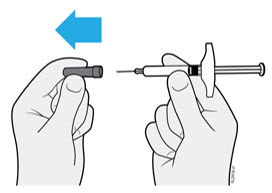
4a Pull the needle cap straight off while holding the syringe body.
- Do not twist or bend the needle cap.
- Never put the needle cap back on. It may damage the needle.
- Do not let anything touch the needle after you remove the needle cap.
- Do not place the uncapped syringe on any surface after you remove the needle cap.
- Do not try to push air bubbles out of the syringe. It is okay to see air bubbles.
- It is normal to see a drop of medicine come out of the needle.
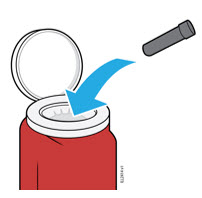
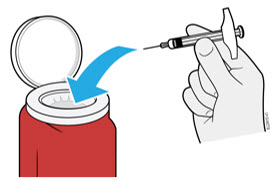
4b Place the needle cap in the sharps disposal container.
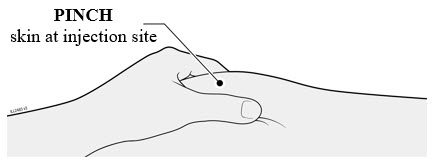
4c Pinch the skin around the injection site before injection.
- Pinch the skin between the thumb and pointer (index) finger to create a bump for the injection.
- If possible, the bump should be about 2 inches wide.
4d Insert the needle into the pinched skin at a 45-degree angle.
- Do not place your finger on the plunger rod while inserting the needle, as this may result in lost medicine.
4e Slowly press the plunger rod all the way down until it reaches the bottom of the syringe to inject the medicine.
- Do not pull back on the plunger rod at any time.
- Do not remove the syringe until all of the medicine has been injected.
5. Disposing of AMJEVITA and Checking the Injection Site
5a Place the used syringe in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the syringe.
5b Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site. Apply an adhesive bandage if necessary.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMJEVITA
Manufactured by:
15PRINCIPAL DISPLAY PANEL - 20 mg Syringe Carton
20 mg/
AMJEVITA™
NDC 55513-411-01
Not made with
For Use in Pediatric Patients
20 mg/0.4 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide is required
Contains 1 Single-dose
CAUTION, Consult
Do not re-use
Rx Only
AMGEN

16PRINCIPAL DISPLAY PANEL - 40 mg Syringe Carton
40
AMJEVITA™
NDC 55513-410-01
Not made with
40 mg/0.8 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide is required
Contains 1 Single-dose
CAUTION, Consult
Do not re-use
Rx Only
AMGEN

17PRINCIPAL DISPLAY PANEL - 40 mg Autoinjector Carton
40 mg/
AMJEVITA™
NDC 55513-400-01
SureClick
Not made with
40 mg/0.8 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Keep out of the sight and reach of children.
ATTENTION: Enclosed Medication Guide is
Contains 1 Single-dose
CAUTION, Consult
Do not re-use
Rx Only
AMGEN

18PRINCIPAL DISPLAY PANEL - 10 mg/0.2 mL Syringe Carton
AMGEN
AMJEVITA™
NDC 55513-413-01
10 mg/
1
10 mg/0.2 mL
Rx Only
Do not use
Not made with
For Use in Pediatric Patients
Refrigerate
CAUTION, Consult

19PRINCIPAL DISPLAY PANEL - 20 mg/0.2 mL Syringe Carton
AMJEVITA™
NDC 55513-399-01
20 mg/
20 mg/0.2 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide is required
Rx Only
Do not use
Not made with
1
For Use in Pediatric Patients
Refrigerate
CAUTION, Consult
AMGEN

20PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Syringe Carton
AMJEVITA™
NDC 55513-479-01
40 mg/
40 mg/0.4 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide is required
Rx Only
Do not use
Not made with
1
Refrigerate
CAUTION, Consult
AMGEN

21PRINCIPAL DISPLAY PANEL - 80 mg Syringe Carton
AMJEVITA™
NDC 55513-480-01
80 mg/
80 mg/0.8 mL
For Subcutaneous Use Only.
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide is
Rx Only
Do not use
Not made with
1
Refrigerate
CAUTION, Consult
AMGEN
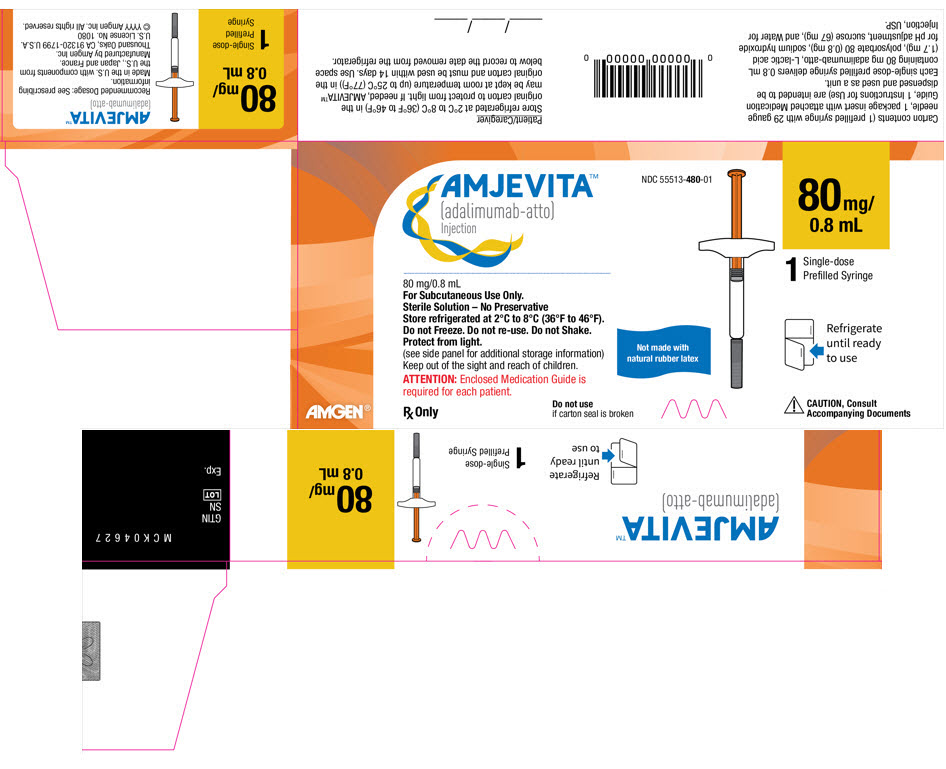
22PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Autoinjector Carton
AMJEVITA™
NDC 55513-482-01
40 mg/
40 mg/0.4 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide
Rx Only
Not made with
1
Refrigerate
CAUTION, Consult
AMGEN

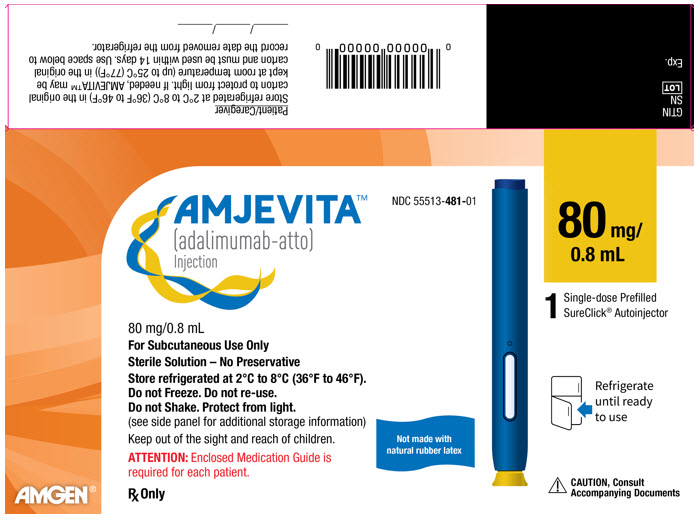
23PRINCIPAL DISPLAY PANEL - 80 mg Autoinjector Carton
AMJEVITA™
NDC 55513-481-01
80 mg/
80 mg/0.8 mL
For Subcutaneous Use Only
Sterile Solution – No Preservative
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ATTENTION: Enclosed Medication Guide is
Rx Only
Not made with
1
Refrigerate
CAUTION, Consult
AMGEN