Generic Name
Sulfasalazine
Brand Names
Azulfidine EN-tabs, Azulfidine
FDA approval date: June 20, 1950
Classification: Aminosalicylate
Form: Tablet
What is Azulfidine EN-tabs (Sulfasalazine)?
Sulfasalazine delayed release tablets are indicated: a) in the treatment of mild to moderate ulcerative colitis, and as adjunctive therapy in severe ulcerative colitis; b) for the prolongation of the remission period between acute attacks of ulcerative colitis; c) in the treatment of patients with rheumatoid arthritis who have responded inadequately to salicylates or other nonsteroidal anti-inflammatory drugs ; and d) in the treatment of pediatric patients with polyarticular-course 1 juvenile rheumatoid arthritis who have responded inadequately to salicylates or other nonsteroidal anti-inflammatory drugs. Sulfasalazine delayed release tablets are particularly indicated in patients with ulcerative colitis who cannot take uncoated sulfasalazine tablets because of gastrointestinal intolerance, and in whom there is evidence that this intolerance is not primarily the result of high blood levels of sulfapyridine and its metabolites, e.g., patients experiencing nausea and vomiting with the first few doses of the drug, or patients in whom a reduction in dosage does not alleviate the adverse gastrointestinal effects. In patients with rheumatoid arthritis or juvenile rheumatoid arthritis, rest and physiotherapy as indicated should be continued. Unlike anti-inflammatory drugs, sulfasalazine delayed release tablets do not produce an immediate response. Concurrent treatment with analgesics and/or nonsteroidal anti-inflammatory drugs is recommended at least until the effect of sulfasalazine delayed release tablets is apparent.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Azulfidine (Sulfasalazine)
1DESCRIPTION
AZULFIDINE EN-tabs Tablets contain sulfasalazine, formulated in a delayed release tablet (enteric-coated), 500 mg, for oral administration.
AZULFIDINE EN-tabs Tablets are film coated with cellulose acetate phthalate to retard disintegration of the tablet in the stomach and reduce potential irritation of the gastric mucosa.
Therapeutic Classification: Anti-inflammatory agent and/or immunomodulatory agent.
Chemical Designation: 5-([p-(2-pyridylsulfamoyl)phenyl]azo) salicylic acid.
Chemical Structure:
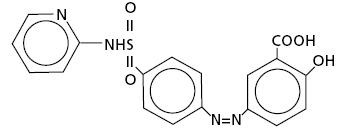
Molecular Formula: C18H14N4O5S
Inactive ingredients: beeswax (white), carnauba wax, cellacefate, magnesium stearate, polyethylene glycol, povidone, propylene glycol, self-emulsifying glycerol monostearate, silica (colloidal anhydrous), starch (pregelatinized), talc.
2INDICATIONS AND USAGE
AZULFIDINE EN-tabs Tablets are indicated:
- in the treatment of mild to moderate ulcerative colitis, and as adjunctive therapy in severe ulcerative colitis;
- for the prolongation of the remission period between acute attacks of ulcerative colitis;
- in the treatment of patients with rheumatoid arthritis who have responded inadequately to salicylates or other nonsteroidal anti-inflammatory drugs (e.g., an insufficient therapeutic response to, or intolerance of, an adequate trial of full doses of one or more nonsteroidal anti-inflammatory drugs); and
- in the treatment of pediatric patients with polyarticular-course
AZULFIDINE EN-tabs is particularly indicated in patients with ulcerative colitis who cannot take uncoated sulfasalazine tablets because of gastrointestinal intolerance, and in whom there is evidence that this intolerance is not primarily the result of high blood levels of sulfapyridine and its metabolites, e.g., patients experiencing nausea and vomiting with the first few doses of the drug, or patients in whom a reduction in dosage does not alleviate the adverse gastrointestinal effects.
In patients with rheumatoid arthritis or juvenile rheumatoid arthritis, rest and physiotherapy as indicated should be continued. Unlike anti-inflammatory drugs, AZULFIDINE EN-tabs does not produce an immediate response. Concurrent treatment with analgesics and/or nonsteroidal anti-inflammatory drugs is recommended at least until the effect of AZULFIDINE EN-tabs is apparent.
3CONTRAINDICATIONS
AZULFIDINE EN-tabs Tablets are contraindicated in:
4ADVERSE REACTIONS
The most common adverse reactions associated with sulfasalazine in ulcerative colitis are anorexia, headache, nausea, vomiting, gastric distress, and apparently reversible oligospermia. These occur in about one-third of the patients. Less frequent adverse reactions are pruritus, urticaria, rash, fever, Heinz body anemia, hemolytic anemia, and cyanosis, which may occur at a frequency of 1 in 30 patients or less. Experience suggests that with a daily dose of 4 g or more, or total serum sulfapyridine levels above 50 µg/mL, the incidence of adverse reactions tends to increase.
Similar adverse reactions are associated with sulfasalazine use in adult rheumatoid arthritis, although there was a greater incidence of some reactions. In rheumatoid arthritis studies, the following common adverse reactions were noted: nausea (19%), dyspepsia (13%), rash (13%), headache (9%), abdominal pain (8%), vomiting (8%), fever (5%), dizziness (4%), stomatitis (4%), pruritis (4%), abnormal liver function tests (4%), leukopenia (3%), and thrombocytopenia (1%). One report
In general, the adverse reactions in juvenile rheumatoid arthritis patients are similar to those seen in patients with adult rheumatoid arthritis except for a high frequency of serum sickness-like syndrome in systemic-course juvenile rheumatoid arthritis (see
Although the listing which follows includes a few adverse reactions which have not been reported with this specific drug, the pharmacological similarities among the sulfonamides require that each of these reactions be considered when AZULFIDINE EN-tabs is administered.
Less common or rare adverse reactions include:
Blood dyscrasias: aplastic anemia, agranulocytosis, megaloblastic (macrocytic) anemia, purpura, hypoprothrombinemia, methemoglobinemia, congenital neutropenia, and myelodysplastic syndrome.
Hypersensitivity reactions: erythema multiforme, epidermal necrolysis (SJS/TEN) with corneal damage, exfoliative dermatitis, DRESS, anaphylaxis, serum sickness syndrome, interstitial lung disease, pneumonitis with or without eosinophilia, vasculitis, fibrosing alveolitis, pleurisy/pleuritis, pericarditis with or without tamponade, allergic myocarditis, polyarteritis nodosa, lupus erythematosus-like syndrome, hepatitis and hepatic necrosis with or without immune complexes, fulminant hepatitis, sometimes leading to liver transplantation, parapsoriasis varioliformis acuta (Mucha-Haberman syndrome), rhabdomyolysis, photosensitization, arthralgia, periorbital edema, conjunctival and scleral injection and alopecia.
Gastrointestinal reactions: hepatitis, hepatic failure, pancreatitis, bloody diarrhea, impaired folic acid absorption, impaired digoxin absorption, stomatitis, diarrhea, abdominal pains, and neutropenic enterocolitis.
Central Nervous System reactions: transverse myelitis, convulsions, meningitis, transient lesions of the posterior spinal column, cauda equina syndrome, Guillain-Barre syndrome, peripheral neuropathy, mental depression, vertigo, hearing loss, insomnia, ataxia, hallucinations, tinnitus, and drowsiness.
Renal reactions: toxic nephrosis with oliguria and anuria, nephritis, nephrotic syndrome, urinary tract infections, hematuria, crystalluria, proteinuria, and hemolytic-uremic syndrome.
Other reactions: urine discoloration and skin discoloration.
The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides), and oral hypoglycemic agents. Goiter production, diuresis and hypoglycemia have occurred rarely in patients receiving sulfonamides.
Cross-sensitivity may exist with these agents. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides and long-term administration has produced thyroid malignancies in this species.
4.1Postmarketing Reports
The following events have been identified during post-approval use of products which contain (or are metabolized to) mesalamine in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to mesalamine:
Blood dyscrasias: pseudomononucleosis
Cardiac disorders: myocarditis
Hepatobiliary disorders: reports of hepatotoxicity, including elevated liver function tests (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, hepatitis cholestatic, cholestasis and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. One case of Kawasaki-like syndrome, which included hepatic function changes, was also reported.
Immune system disorders: anaphylaxis
Metabolism and nutrition system disorders: folate deficiency
Renal and urinary disorders: nephrolithiasis
Respiratory, thoracic and mediastinal disorders: oropharyngeal pain
Skin and subcutaneous tissue disorders: angioedema, purpura, SJS/TEN, DRESS, and AGEP
Vascular disorders: pallor
5DRUG ABUSE AND DEPENDENCE
None reported.
6OVERDOSAGE
There is evidence that the incidence and severity of toxicity following overdosage is directly related to the total serum sulfapyridine concentration. Symptoms of overdosage may include nausea, vomiting, gastric distress and abdominal pains. In more advanced cases, central nervous system symptoms such as drowsiness, convulsions, etc., may be observed. Serum sulfapyridine concentrations may be used to monitor the progress of recovery from overdosage.
There are no documented reports of deaths due to ingestion of large single doses of sulfasalazine. It has not been possible to determine the LD
6.1Instructions for Overdosage
Gastric lavage or emesis plus catharsis as indicated. Alkalinize urine. If kidney function is normal, force fluids. If anuria is present, restrict fluids and salt, and treat appropriately. Catheterization of the ureters may be indicated for complete renal blockage by crystals. The low molecular weight of sulfasalazine and its metabolites may facilitate their removal by dialysis.
7DOSAGE AND ADMINISTRATION
The dosage of AZULFIDINE EN-tabs Tablets should be adjusted to each individual's response and tolerance.
Patients should be instructed to take AZULFIDINE EN-tabs in evenly divided doses, preferably after meals, and to swallow the tablets whole.
7.1Adult Rheumatoid Arthritis
2 g daily in two evenly divided doses. It is advisable to initiate therapy with a lower dosage of AZULFIDINE EN-tabs, e.g., 0.5 to 1.0 g daily, to reduce possible gastrointestinal intolerance. A suggested dosing schedule is given below.
In rheumatoid arthritis, the effect of AZULFIDINE EN-tabs can be assessed by the degree of improvement in the number and extent of actively inflamed joints. A therapeutic response has been observed as early as 4 weeks after starting treatment with AZULFIDINE EN-tabs, but treatment for 12 weeks may be required in some patients before clinical benefit is noted. Consideration can be given to increasing the daily dose of AZULFIDINE EN-tabs to 3 g if the clinical response after 12 weeks is inadequate. Careful monitoring is recommended for doses over 2 g per day.
Suggested Dosing Schedule for Adult Rheumatoid Arthritis:
7.2Juvenile Rheumatoid Arthritis - polyarticular course
Children, six years of age and older: 30 to 50 mg/kg of body weight daily in two evenly divided doses. Typically, the maximum dose is 2 g per day. To reduce possible gastrointestinal intolerance, begin with a quarter to a third of the planned maintenance dose and increase weekly until reaching the maintenance dose at one month.
Some patients may be sensitive to treatment with sulfasalazine. Various desensitization-like regimens have been reported to be effective in 34 of 53 patients,
8HOW SUPPLIED
AZULFIDINE EN-tabs Tablets, 500 mg, are elliptical, gold-colored, film enteric-coated tablets, monogrammed "102" on one side and "KPh" on the other. They are available in the following package sizes:
8.1Storage
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
9REFERENCES
- van Rossum MAJ, et al. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Arth Rheum 1998;41:808–816.
- Mogadam M, et al. Pregnancy in inflammatory bowel disease: effect of sulfasalazine and corticosteroids on fetal outcome. Gastroenterology 1981;80:726.
- Kaufman DW, editor. Birth defects and drugs during pregnancy. Littleton, MA: Publishing Sciences Group, Inc., 1977:296–313.
- Jarnerot G. Fertility, sterility and pregnancy in chronic inflammatory bowel disease. Scand J Gastroenterol 1982;17:1–4.
- Imundo LF, Jacobs JC. Sulfasalazine therapy for juvenile rheumatoid arthritis. J Rheumatol 1996;23:360–366.
- Hertzberger-ten Cate R, Cats A. Toxicity of sulfasalazine in systemic juvenile chronic arthritis. Clin Exp Rheumatol 1991;9:85–8.
- Farr M, et al. Immunodeficiencies associated with sulphasalazine therapy in inflammatory arthritis. British Jnl Rheum 1991;30:413–417.
- Korelitz B, et al. Desensitization to sulfasalazine in allergic patients with IBD: an important therapeutic modality. Gastroenterology 1982;82:1104.
- Holdworth CG. Sulphasalazine desensitization. Br Med J 1981;282:110.
- Taffet SL, Das KM. Desensitization of patients with inflammatory bowel disease to sulfasalazine. Am J Med 1982;73:520–4.
10PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
Pfizer
NDC 0013-0102-01
Azulfidine EN-tabs
sulfasalazine delayed release
tablets, USP
tablets, USP
500 mg
100 Enteric-coated Tablets

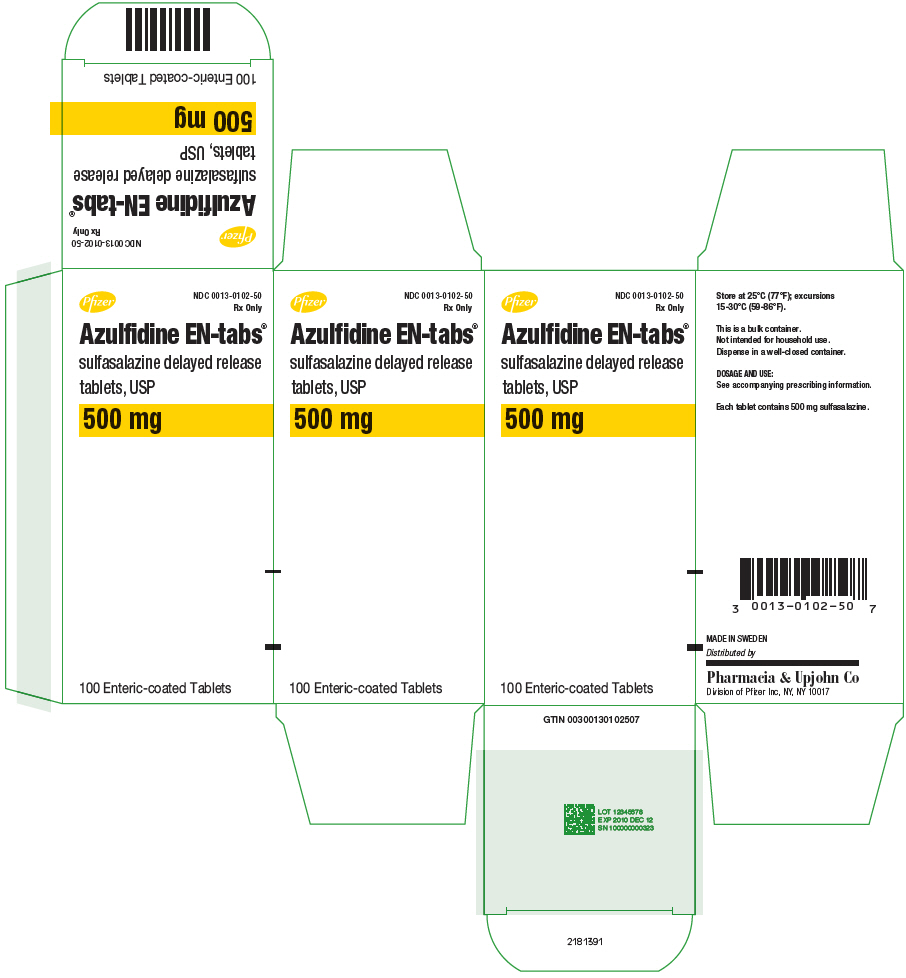
11PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label - NDC 0013-0102-50
Pfizer
NDC 0013-0102-50
Azulfidine EN-tabs
sulfasalazine delayed release
500 mg
100 Enteric-coated Tablets

12PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton - NDC 0013-0102-50
Pfizer
NDC 0013-0102-50
Azulfidine EN-tabs
sulfasalazine delayed release
500 mg
100 Enteric-coated Tablets