Brand Name
Valtrex
Generic Name
ValACYclovir
View Brand Information FDA approval date: August 01, 1995
Classification: Herpes Zoster Virus Nucleoside Analog DNA Polymerase Inhibitor
Form: Tablet
What is Valtrex (ValACYclovir)?
Valacyclovir tablet is a deoxynucleoside analogue DNA polymerase inhibitor indicated for: Adult Patients.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
VALTREX (valacyclovir hydrochloride)
1DOSAGE AND ADMINISTRATION
- VALTREX may be given without regard to meals.
- Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) may be prepared extemporaneously from 500-mg VALTREX tablets for use in pediatric patients for whom a solid dosage form is not appropriate
1.1Adult Dosing Recommendations
Cold Sores (Herpes Labialis)
The recommended dosage of VALTREX for treatment of cold sores is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning).
Genital Herpes
Initial Episode:The recommended dosage of VALTREX for treatment of initial genital herpes is 1 gram twice daily for 10 days. Therapy was most effective when administered within 48 hours of the onset of signs and symptoms.
Recurrent Episodes:The recommended dosage of VALTREX for treatment of recurrent genital herpes is 500 mg twice daily for 3 days. Initiate treatment at the first sign or symptom of an episode.
Suppressive Therapy:The recommended dosage of VALTREX for chronic suppressive therapy of recurrent genital herpes is 1 gram once daily in patients with normal immune function. In patients with a history of 9 or fewer recurrences per year, an alternative dose is 500 mg once daily.
In HIV‑1−infected patients with a CD4+ cell count greater than or equal to 100 cells/mm
Reduction of Transmission:The recommended dosage of VALTREX for reduction of transmission of genital herpes in patients with a history of 9 or fewer recurrences per year is 500 mg once daily for the source partner.
Herpes Zoster
The recommended dosage of VALTREX for treatment of herpes zoster is 1 gram 3 times daily for 7 days. Therapy should be initiated at the earliest sign or symptom of herpes zoster and is most effective when started within 48 hours of the onset of rash.
1.2Pediatric Dosing Recommendations
Cold Sores (Herpes Labialis)
The recommended dosage of VALTREX for the treatment of cold sores in pediatric patients aged greater than or equal to 12 years is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning).
Chickenpox
The recommended dosage of VALTREX for treatment of chickenpox in immunocompetent pediatric patients aged 2 to less than 18 years is 20 mg/kg administered 3 times daily for 5 days. The total dose should not exceed 1 gram 3 times daily. Therapy should be initiated at the earliest sign or symptom
1.3Extemporaneous Preparation of Oral Suspension
Ingredients and Preparation per USP‑ NF
VALTREX tablets 500 mg, cherry flavor, and Suspension Structured Vehicle USP-NF (SSV). Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) should be prepared in lots of 100 mL.
Instructions for Preparing Suspension at Time of Dispensing
- Prepare SSV according to the USP-NF.
- Using a pestle and mortar, grind the required number of VALTREX 500-mg tablets until a fine powder is produced (5 VALTREX tablets for 25-mg/mL suspension; 10 VALTREX tablets for 50-mg/mL suspension).
- Gradually add approximately 5-mL aliquots of SSV to the mortar and triturate the powder until a paste has been produced. Ensure that the powder has been adequately wetted.
- Continue to add approximately 5-mL aliquots of SSV to the mortar, mixing thoroughly between additions, until a concentrated suspension is produced, to a minimum total quantity of 20 mL SSV and a maximum total quantity of 40 mL SSV for both the 25-mg/mL and 50-mg/mL suspensions.
- Transfer the mixture to a suitable 100-mL measuring flask.
- Transfer the cherry flavor* to the mortar and dissolve in approximately 5 mL of SSV. Once dissolved, add to the measuring flask.
- Rinse the mortar at least 3 times with approximately 5-mL aliquots of SSV, transferring the rinsing to the measuring flask between additions.
- Make the suspension to volume (100 mL) with SSV and shake thoroughly to mix.
- Transfer the suspension to an amber glass medicine bottle with a child-resistant closure.
- The prepared suspension should be labeled with the following information “Shake well before using. Store suspension between 2°C to 8°C (36°F to 46°F) in a refrigerator. Discard after 28 days.”
*The amount of cherry flavor added is as instructed by the suppliers of the cherry flavor.
1.4Patients with Renal Impairment
Dosage recommendations for adult patients with reduced renal function are provided in
Hemodialysis
Patients requiring hemodialysis should receive the recommended dose of VALTREX after hemodialysis. During hemodialysis, the half-life of acyclovir after administration of VALTREX is approximately 4 hours. About one-third of acyclovir in the body is removed by dialysis during a 4-hour hemodialysis session.
Peritoneal Dialysis
There is no information specific to administration of VALTREX in patients receiving peritoneal dialysis. The effect of chronic ambulatory peritoneal dialysis (CAPD) and continuous arteriovenous hemofiltration/dialysis (CAVHD) on acyclovir pharmacokinetics has been studied. The removal of acyclovir after CAPD and CAVHD is less pronounced than with hemodialysis, and the pharmacokinetic parameters closely resemble those observed in patients with end-stage renal disease (ESRD) not receiving hemodialysis. Therefore, supplemental doses of VALTREX should not be required following CAPD or CAVHD.
2DOSAGE FORMS AND STRENGTHS
Tablets:
- 500-mg: Each blue, film‑coated, capsule‑shaped tablet printed with “VALTREX 500 mg” contains 556.2 mg of valacyclovir hydrochloride equivalent to 500 mg of the free base.
- 1-gram: Each blue, film‑coated, capsule‑shaped tablet, with a partial scorebar on both sides, printed with “VALTREX 1 gram” contains 1.112 grams of valacyclovir hydrochloride equivalent to 1 gram of the free base.
3CONTRAINDICATIONS
VALTREX is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valacyclovir, acyclovir, or any component of the formulation
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome
- Acute Renal Failure
- Central Nervous System Effects
The most common adverse reactions reported in at least 1 indication by greater than 10% of adult subjects treated with VALTREX and observed more frequently with VALTREX compared with placebo are headache, nausea, and abdominal pain. The only adverse reaction reported in greater than 10% of pediatric subjects aged less than 18 years was headache.
4.1Clinical Trials Experience in Adult Subjects
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cold Sores (Herpes Labialis)
In clinical trials for the treatment of cold sores, the adverse reactions reported by subjects receiving VALTREX 2 grams twice daily (n = 609) or placebo (n = 609) for 1 day, respectively, included headache (14%, 10%) and dizziness (2%, 1%). The frequencies of abnormal ALT (greater than 2 x ULN) were 1.8% for subjects receiving VALTREX compared with 0.8% for placebo. Other laboratory abnormalities (hemoglobin, white blood cells, alkaline phosphatase, and serum creatinine) occurred with similar frequencies in the 2 groups.
Genital Herpes
Initial Episode:In a clinical trial for the treatment of initial episodes of genital herpes, the adverse reactions reported by greater than or equal to 5% of subjects receiving VALTREX 1 gram twice daily for 10 days (n = 318) or oral acyclovir 200 mg 5 times daily for 10 days (n = 318), respectively, included headache (13%, 10%) and nausea (6%, 6%). For the incidence of laboratory abnormalities see Table 2.
Recurrent Episodes:In 3 clinical trials for the episodic treatment of recurrent genital herpes, the adverse reactions reported by greater than or equal to 5% of subjects receiving VALTREX 500 mg twice daily for 3 days (n = 402), VALTREX 500 mg twice daily for 5 days (n = 1,136), or placebo (n = 259), respectively, included headache (16%, 11%, 14%) and nausea (5%, 4%, 5%). For the incidence of laboratory abnormalities see Table 2.
Suppressive Therapy: Suppression of Recurrent Genital Herpes in Immunocompetent Adults:In a clinical trial for the suppression of recurrent genital herpes infections, the adverse reactions reported by subjects receiving VALTREX 1 gram once daily (n = 269), VALTREX 500 mg once daily (n = 266), or placebo (n = 134), respectively, included headache (35%, 38%, 34%), nausea (11%, 11%, 8%), abdominal pain (11%, 9%, 6%), dysmenorrhea (8%, 5%, 4%), depression (7%, 5%, 5%), arthralgia (6%, 5%, 4%), vomiting (3%, 3%, 2%), and dizziness (4%, 2%, 1%). For the incidence of laboratory abnormalities see Table 2.
Suppression of Recurrent Genital Herpes in HIV-1–Infected Subjects:In HIV-1 –infected subjects, frequently reported adverse reactions for VALTREX (500 mg twice daily; n = 194, median days on therapy = 172) and placebo (n = 99, median days on therapy = 59), respectively, included headache (13%, 8%), fatigue (8%, 5%), and rash (8%, 1%). Post-randomization laboratory abnormalities that were reported more frequently in valacyclovir subjects versus placebo included elevated alkaline phosphatase (4%, 2%), elevated ALT (14%, 10%), elevated AST (16%, 11%), decreased neutrophil counts (18%, 10%), and decreased platelet counts (3%, 0%), respectively.
Reduction of Transmission:In a clinical trial for the reduction of transmission of genital herpes, the adverse reactions reported by subjects receiving VALTREX 500 mg once daily (n = 743) or placebo once daily (n = 741), respectively, included headache (29%, 26%), nasopharyngitis (16%, 15%), and upper respiratory tract infection (9%, 10%).
Herpes Zoster
In 2 clinical trials for the treatment of herpes zoster, the adverse reactions reported by subjects receiving VALTREX 1 gram 3 times daily for 7 to 14 days (n = 967) or placebo (n = 195), respectively, included nausea (15%, 8%), headache (14%, 12%), vomiting (6%, 3%), dizziness (3%, 2%), and abdominal pain (3%, 2%). For the incidence of laboratory abnormalities see
4.2Clinical Trials Experience in Pediatric Subjects
The safety profile of VALTREX has been studied in 177 pediatric subjects aged 1 month to less than 18 years. Sixty-five of these pediatric subjects, aged 12 to less than 18 years, received oral tablets for 1 to 2 days for treatment of cold sores. The remaining 112 pediatric subjects, aged 1 month to less than 12 years, participated in 3 pharmacokinetic and safety trials and received valacyclovir oral suspension. Fifty-one of these 112 pediatric subjects received oral suspension for 3 to 6 days. The frequency, intensity, and nature of clinical adverse reactions and laboratory abnormalities were similar to those seen in adults.
Pediatric Subjects Aged 12 to Less than 18 Years (Cold Sores)
In clinical trials for the treatment of cold sores, the adverse reactions reported by adolescent subjects receiving VALTREX 2 grams twice daily for 1 day, or VALTREX 2 grams twice daily for 1 day followed by 1 gram twice daily for 1 day (n = 65, across both dosing groups), or placebo (n = 30), respectively, included headache (17%, 3%) and nausea (8%, 0%).
Pediatric Subjects Aged 1 Month to Less than 12 Years
Adverse events reported in more than 1 subject across the 3 pharmacokinetic and safety trials in children aged 1 month to less than 12 years were diarrhea (5%), pyrexia (4%), dehydration (2%), herpes simplex (2%), and rhinorrhea (2%). No clinically meaningful changes in laboratory values were observed.
4.3Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postmarketing use of VALTREX. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to VALTREX.
General
Facial edema, hypertension, tachycardia.
Allergic
Acute hypersensitivity reactions including anaphylaxis, angioedema, dyspnea, pruritus, rash, and urticaria
Central Nervous System (CNS) Symptoms
Aggressive behavior; agitation; ataxia; coma; confusion; decreased consciousness; dysarthria; encephalopathy; mania; and psychosis, including auditory and visual hallucinations, seizures, tremors
Eye
Visual abnormalities.
Gastrointestinal
Diarrhea.
Hepatobiliary Tract and Pancreas
Liver enzyme abnormalities, hepatitis.
Renal
Renal failure, renal pain (may be associated with renal failure)
Hematologic
Thrombocytopenia, aplastic anemia, leukocytoclastic vasculitis, TTP/HUS
Skin
Erythema multiforme, rashes including photosensitivity, alopecia.
5DRUG INTERACTIONS
No clinically significant drug-drug or drug-food interactions with VALTREX are known
6OVERDOSAGE
Caution should be exercised to prevent inadvertent overdose
7DESCRIPTION
VALTREX (valacyclovir hydrochloride) is the hydrochloride salt of the
VALTREX tablets are for oral administration. Each tablet contains 556.2 mg or 1.112 grams of valacyclovir hydrochloride equivalent to 500 mg or 1 gram of valacyclovir, respectively, and the inactive ingredients carnauba wax, colloidal silicon dioxide, crospovidone, FD&C Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide. The blue, film‑coated tablets are printed with edible white ink.
The chemical name of valacyclovir hydrochloride is
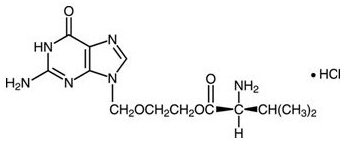
Valacyclovir hydrochloride is a white to off-white powder with the molecular formula C
8HOW SUPPLIED/STORAGE AND HANDLING
VALTREX tablets (blue, film‑coated, capsule‑shaped tablets printed with “VALTREX 500 mg”) containing 556.2 mg of valacyclovir hydrochloride equivalent to 500 mg valacyclovir.
Bottle of 30 (NDC 0173-0933-08).
Bottle of 90 (NDC 0173-0933-10).
VALTREX tablets (blue, film‑coated, capsule‑shaped tablets, with a partial scorebar on both sides, printed with “VALTREX 1 gram”) containing 1.112 grams of valacyclovir hydrochloride equivalent to 1 gram of valacyclovir.
Bottle of 30 (NDC 0173-0565-04).
Bottle of 90 (NDC 0173-0565-10).
Storage:
Store at 15°C to 25°C (59°F to 77°F). Dispense in a well-closed container as defined in the USP.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Importance of Adequate Hydration
Patients should be advised to maintain adequate hydration.
Missed Dose
Instruct patients that if they miss a dose of VALTREX, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose.
Cold Sores (Herpes Labialis)
Patients should be advised to initiate treatment at the earliest symptom of a cold sore (e.g., tingling, itching, or burning). There are no data on the effectiveness of treatment initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer). Patients should be instructed that treatment for cold sores should not exceed 1 day (2 doses) and that their doses should be taken about 12 hours apart. Patients should be informed that VALTREX is not a cure for cold sores.
Genital Herpes
Patients should be informed that VALTREX is not a cure for genital herpes. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes is frequently transmitted in the absence of symptoms through asymptomatic viral shedding. Therefore, patients should be counseled to use safer sex practices in combination with suppressive therapy with VALTREX. Sex partners of infected persons should be advised that they might be infected even if they have no symptoms. Type‑specific serologic testing of asymptomatic partners of persons with genital herpes can determine whether risk for HSV‑2 acquisition exists.
VALTREX has not been shown to reduce transmission of sexually transmitted infections other than HSV‑2.
If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
There are no data on the effectiveness of treatment initiated more than 72 hours after the onset of signs and symptoms of a first episode of genital herpes or more than 24 hours after the onset of signs and symptoms of a recurrent episode.
There are no data on the safety or effectiveness of chronic suppressive therapy of more than 1 year’s duration in otherwise healthy patients. There are no data on the safety or effectiveness of chronic suppressive therapy of more than 6 months’ duration in HIV-1−infected patients.
Herpes Zoster
There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Chickenpox
Patients should be advised to initiate treatment at the earliest sign or symptom of chickenpox.
Trademarks are owned by or licensed to the GSK group of companies.
Distributed by:
GlaxoSmithKline
Durham, NC 27701
©2022 GSK group of companies or its licensor.
VTX: 11PI
PHARMACIST‑DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _