Brand Name
ConZip
Generic Name
TraMADol
View Brand Information FDA approval date: June 19, 2002
Classification: Opioid Agonist
Form: Tablet, Capsule, Solution
What is ConZip (TraMADol)?
Tramadol hydrochloride tablets are indicated in adults for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Limitations of Use Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses [see WARNINGS AND PRECAUTIONS.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ConZip (tramadol hydrochloride)
1INDICATIONS AND USAGE
CONZIP is indicated for the management of severe and persistent pain that requires an extended treatment period with a daily opioid analgesic and for which alternative treatment options are inadequate.
2DOSAGE FORMS AND STRENGTHS
Extended-release capsules are available as:
100 mg Capsules: White capsule imprinted with blue ink "G 252" on cap and "100" between lines on the body
3CONTRAINDICATIONS
CONZIP is contraindicated for:
- All children younger than 12 years of age
- Postoperative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy
CONZIP is also contraindicated in patients with:
- Significant respiratory depression
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment
- Known or suspected gastrointestinal obstruction, including paralytic ileus
- Hypersensitivity to tramadol (e.g., anaphylaxis)
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use within the last 14 days
4ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are described in greater detail, in other sections:
- Addiction, Abuse, and Misuse
- Life-Threatening Respiratory Depression
- Interactions with Benzodiazepines and Other CNS Depressants
- Neonatal Opioid Withdrawal Syndrome
- Ultra-Rapid Metabolism of Tramadol and Other Risk Factors for Life-Threatening Respiratory Depression in Children
- Opioid-Induced Hyperalgesia and Allodynia
- Serotonin Syndrome
- Seizures
- Suicide
- Adrenal Insufficiency
- Severe Hypotension
- Gastrointestinal Adverse Reactions
- Hypersensitivity Reactions
- Withdrawal
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
CONZIP capsules were administered to a total of 1987 patients in clinical trials. These included four double-blind and one long-term, open-label study in patients with osteoarthritis of the hip and knee. A total of 812 patients were 65 years or older. Adverse reactions with doses from 100 mg to 300 mg in the four pooled, randomized, double-blind, placebo-controlled studies in patients with chronic non-malignant pain are presented in the following table (see
The following adverse reactions were reported from all chronic pain studies (N=1917). The lists below include adverse reactions not otherwise noted in Table 1.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of tramadol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
5DRUG INTERACTIONS
Table 2 includes clinically significant drug interactions with CONZIP.
6DESCRIPTION
CONZIP (tramadol hydrochloride) is an opioid agonist in an extended-release oral formulation. The chemical name for tramadol hydrochloride USP is (±)
Figure 1
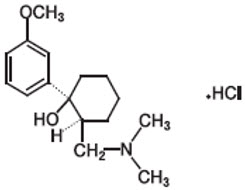
The molecular weight of tramadol hydrochloride USP is 299.8. It is a white, bitter, crystalline and odorless powder that is readily soluble in water and ethanol and has a pKa of 9.41. The n-octanol/water log partition coefficient (logP) is 1.35 at pH 7.
CONZIP capsules contain a total dose of tramadol hydrochloride 100, 200, and 300 mg in a combination of immediate-release and extended-release components.
CONZIP capsules are white in color. Inactive ingredients include gelatin, titanium dioxide, shellac, FD & C Blue #2 aluminum lake (E132) (100 and 200 mg capsules), D & C Red #7 calcium lake (E180) (200 and 300 mg capsules), D & C Yellow #10 aluminum lake (300 mg capsule), lactose monohydrate 200 mesh, microcrystalline cellulose, povidone K30, corn starch, sodium starch glycolate, magnesium stearate, sucrose stearate, hypromellose, talc, polysorbate 80, Eudragit NE 30D, and simethicone emulsion.
7CLINICAL STUDIES
CONZIP is bioequivalent under fasting conditions to another extended-release tramadol product
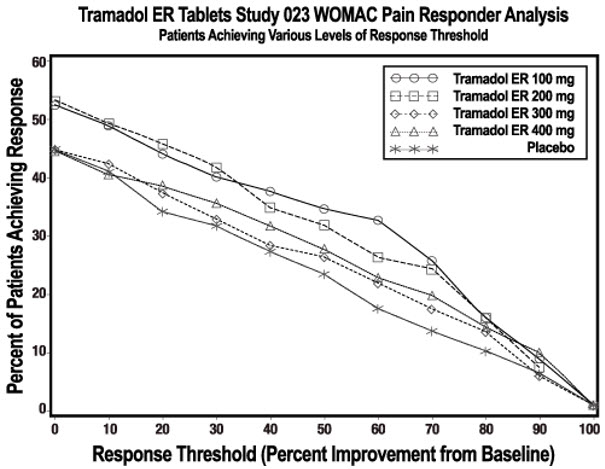
In one 12-week randomized, double-blind, placebo-controlled study, patients with moderate to moderately severe pain due to osteoarthritis of the knee and/or hip were administered doses from 100 mg to 400 mg daily. Treatment with the extended-release tramadol product was initiated at 100 mg once daily for four days then increased by 100 mg per day increments every five days to the randomized fixed dose. Between 51% and 59% of patients in active treatment groups completed the study and 56% of patients in the placebo group completed the study. Discontinuations due to adverse events were more common in the extended-release tramadol product 200 mg, 300 mg and 400 mg treatment groups (20%, 27%, and 30% of discontinuations, respectively) compared to 14% of the patients treated with the extended-release tramadol product 100 mg and 10% of patients treated with placebo.
Pain, as assessed by the WOMAC Pain subscale, was measured at 1, 2, 3, 6, 9, and 12 weeks and change from baseline assessed. A responder analysis based on the percent change in WOMAC Pain subscale demonstrated a statistically significant improvement in pain for the 100 mg and 200 mg treatment groups compared to placebo (see
Figure 2
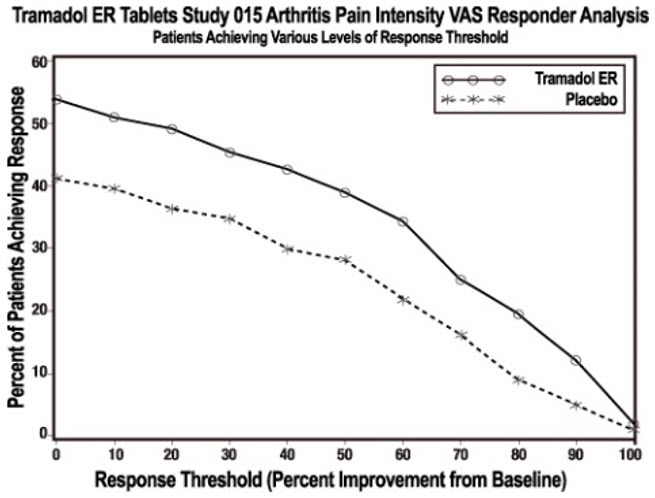
In one 12-week randomized, double-blind, placebo-controlled flexible-dosing trial of the extended-release tramadol product in patients with osteoarthritis of the knee, patients titrated to an average daily dose of approximately 270 mg/day. Forty-nine percent of patients randomized to the active treatment group completed the study, while 52% of patients randomized to placebo completed the study. Most of the early discontinuations in the active treatment group were due to adverse events, accounting for 27% of the early discontinuations in contrast to 7% of the discontinuations from the placebo group. Thirty-seven percent of the placebo-treated patients discontinued the study due to lack of efficacy compared to 15% of active-treated patients. The active treatment group demonstrated a statistically significant decrease in the mean Visual Analog Scale (VAS) score, and a statistically significant difference in the responder rate, based on the percent change from baseline in the VAS score, measured at 1, 2, 4, 8, and 12 weeks, between patients receiving the extended-release tramadol product and placebo (see
Figure 3
Four randomized, placebo-controlled clinical trials of CONZIP were conducted, none of which demonstrated efficacy but which differed in design from the preceding clinical studies described. Two trials were 12-week randomized placebo-controlled trials of CONZIP 100 mg/day, 200 mg/day, and 300 mg/day versus placebo in patients with moderate to moderately severe osteoarthritis pain of the hip and knee. The other two 12 week trials were similar in design, but only studied CONZIP 300 mg/day. In this fixed-dose design, subjects were required to titrate to a fixed dose, even if their pain responded to a lower titration dose.
8HOW SUPPLIED/STORAGE AND HANDLING
CONZIP (tramadol hydrochloride) capsules are supplied as opaque white hard gelatin capsules, imprinted as follows.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
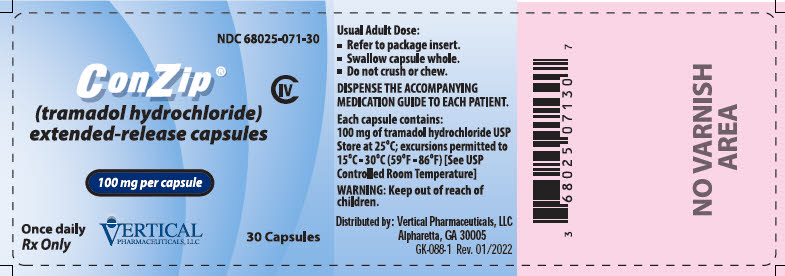
10PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
NDC 68025-071-30
ConZip
CIV
100 mg per capsule
Once daily
VERTICAL
30 Capsules
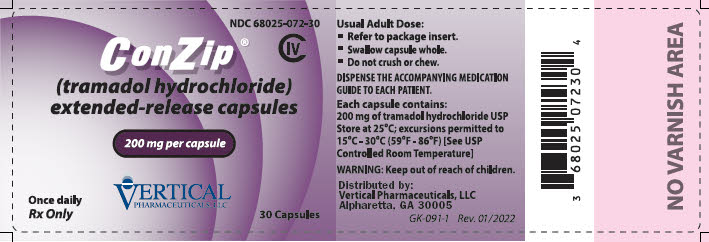
11PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle Label
NDC 68025-072-30
ConZip
CIV
200 mg per capsule
Once daily
VERTICAL
30 Capsules
12PRINCIPAL DISPLAY PANEL - 300 mg Capsule Bottle Label
NDC 68025-073-30
ConZip
CIV
300 mg per capsule
Once daily
VERTICAL
30 Capsules