Brand Name
Elidel
Generic Name
Pimecrolimus
View Brand Information FDA approval date: December 02, 2001
Classification: Calcineurin Inhibitor Immunosuppressant
Form: Cream
What is Elidel (Pimecrolimus)?
INDICATION AND USAGE Pimecrolimus cream, 1% is indicated as second-line therapy for the short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adults and children 2 years of age and older, who have failed to respond adequately to other topical prescription treatments, or when those treatments are not advisable. Pimecrolimus cream, 1% is not indicated for use in children less than 2 years of age [see Warnings and Precautions.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 3-arm, Multinational, Multicenter Study to Evaluate the Efficacy and Safety of Amlitelimab by Subcutaneous Injection in Participants Aged 12 Years and Older With Moderate-to-severe Atopic Dermatitis (AD) Who Are on Background Topical Corticosteroids and Have Had an Inadequate Response to Prior Biologic Therapy or Oral Janus Kinase (JAK) Inhibitor Treatment
Summary: This is a parallel group, Phase 3, multinational, multicenter, randomized, double-blind, placebo-controlled, 3-arm study for treatment of participants diagnosed with moderate-to-severe AD on background TCS who have had inadequate response to prior biologic or oral JAKi therapy. The purpose of this study is to measure the efficacy and safety of treatment with amlitelimab solution for subcutaneous (...
Related Latest Advances
Brand Information
ELIDEL (Pimecrolimus)
WARNING: LONG-TERM SAFETY OF TOPICAL CALCINEURIN INHIBITORS HAS NOT BEEN ESTABLISHED
Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including ELIDEL Cream, 1%.
Therefore:
Therefore:
- Continuous long-term use of topical calcineurin inhibitors, including ELIDEL Cream, 1%, in any age group should be avoided and application limited to areas of involvement with atopic dermatitis
- ELIDEL Cream, 1% is not indicated for use in children less than 2 years of age
1INDICATION AND USAGE
ELIDEL
ELIDEL Cream, 1% is not indicated for use in children less than 2 years of age[see .
2DOSAGE AND ADMINISTRATION
Apply a thin layer of ELIDEL Cream, 1% to the affected skin twice daily. The patient should stop using ELIDEL Cream, 1% when signs and symptoms (e.g., itch, rash and redness) resolve and should be instructed on what actions to take if symptoms recur.
If signs and symptoms persist beyond 6 weeks, patients should be re-examined by their healthcare provider to confirm the diagnosis of atopic dermatitis.
Continuous long-term use of ELIDEL Cream, 1% should be avoided, and application should be limited to areas of involvement with atopic dermatitis
The safety of ELIDEL Cream, 1% under occlusion, which may promote systemic exposure, has not been evaluated. Avoid use of ELIDEL Cream, 1% with occlusive dressings.
3DOSAGE FORMS AND STRENGTHS
Cream, 1%.
Each gram of ELIDEL Cream, 1% contains 10 mg of pimecrolimus in a whitish cream base.
4CONTRAINDICATIONS
ELIDEL Cream, 1% is contraindicated in individuals with a history of hypersensitivity to pimecrolimus or any of the components of the cream.
5DRUG INTERACTIONS
Potential interactions between ELIDEL Cream, 1% and other drugs, including immunizations, have not been systematically evaluated. Due to low blood levels of pimecrolimus detected in some patients after topical application, systemic drug interactions are not expected, but cannot be ruled out. The concomitant administration of known CYP3A family of inhibitors in patients with widespread and/or erythrodermic disease should be done with caution. Some examples of such drugs are erythromycin, itraconazole, ketoconazole, fluconazole, calcium channel blockers and cimetidine.
6DESCRIPTION
ELIDEL Cream, 1%, for topical use, contains the compound pimecrolimus, the immunosuppressant 33-epi-chloro-derivative of the macrolactam ascomycin.
Chemically, pimecrolimus is (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-12-[(1E)-2-{(1R,3R,4S)-4-chloro-3-methoxycyclohexyl}-1-methylvinyl]-17-ethyl-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone. The compound has the empirical formula C
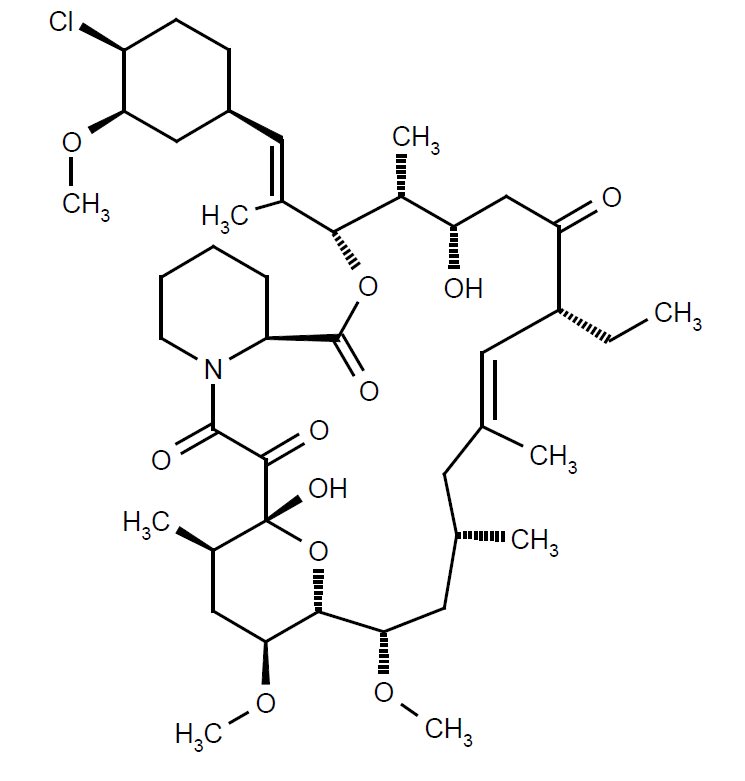
Pimecrolimus is a white to off-white fine crystalline powder. It is soluble in methanol and ethanol and insoluble in water.
Each gram of ELIDEL Cream, 1% contains 10 mg of pimecrolimus in a whitish cream base of benzyl alcohol, cetyl alcohol, citric acid anhydrous, mono- and diglycerides, oleyl alcohol, propylene glycol, sodium cetostearyl sulphate, sodium hydroxide, stearyl alcohol, triglycerides and water.
7CLINICAL STUDIES
Three randomized, double-blind, vehicle-controlled, multi-center, Phase 3 trials were conducted in 589 pediatric subjects ages 3 months-17 years old to evaluate ELIDEL Cream, 1% for the treatment of mild to moderate atopic dermatitis. Two of the three trials support the use of ELIDEL Cream, 1% in subjects 2 years and older with mild to moderate atopic dermatitis
Two identical 6-week, randomized, vehicle-controlled, multi-center, Phase 3 trials were conducted to evaluate ELIDEL Cream, 1% for the treatment of mild to moderate atopic dermatitis. A total of 403 pediatric subjects 2-17 years old were included in the trials. The male/female ratio was approximately 50% and 29% of the subjects were African American. At trial entry, 59% of subjects had moderate disease and the mean body surface area (BSA) affected was 26%. About 75% of subjects had atopic dermatitis affecting the face and/or neck region. In these trials, subjects applied either ELIDEL Cream, 1% or vehicle cream twice daily to 5% to 96% of their BSA for up to 6 weeks. At endpoint, based on the physician’s global evaluation of clinical response, 35% of subjects treated with ELIDEL Cream, 1% were clear or almost clear of signs of atopic dermatitis compared to only 18% of vehicle-treated subjects. More ELIDEL subjects (57%) had mild or no pruritus at 6 weeks compared to vehicle subjects (34%). The improvement in pruritus occurred in conjunction with the improvement of the subjects’ atopic dermatitis.
In these two 6-week trials of ELIDEL Cream, 1%, the combined efficacy results at endpoint are presented in Table 2 as follows:
In the two pediatric trials that independently support the use of ELIDEL Cream, 1% in mild to moderate atopic dermatitis, a significant treatment effect was seen by day 15. Of the key signs of atopic dermatitis, erythema, infiltration/papulation, lichenification, and excoriations were reduced at day 8 when compared to vehicle.
Figure 1 depicts the time course of improvement in the percent body surface area affected as a result of treatment with ELIDEL Cream, 1% in 2-17 year olds.
Figure 1
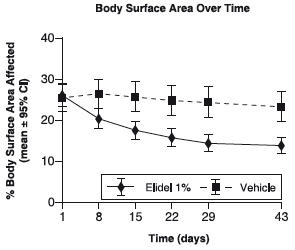
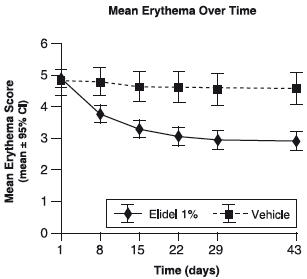
Figure 2 shows the time course of improvement in erythema as a result of treatment with ELIDEL Cream, 1% in 2-17 year olds.
Figure 2
8HOW SUPPLIED/STORAGE AND HANDLING
ELIDEL Cream, 1% is a whitish cream available in tubes of 30 grams, 60 grams, and 100 grams.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Patients using ELIDEL Cream, 1% should receive the following information and instructions:
- ELIDEL Cream, 1% may cause serious side effects. It is not known if ELIDEL Cream, 1% is safe to use for a long period of time. A very small number of people who have used ELIDEL Cream, 1% have had cancer (for example, skin or lymphoma). However, a link with ELIDEL Cream, 1% use has not been shown. Because of this concern:
- A patient should not use ELIDEL Cream, 1% continuously for a long time.
- ELIDEL Cream, 1% should be used only on areas of skin that have eczema.
- ELIDEL Cream, 1% is not for use on a child under 2 years old.
- A patient should not use sun lamps, tanning beds, or get treatment with ultraviolet light therapy during treatment with ELIDEL Cream, 1%.
- A patient should limit sun exposure during treatment with ELIDEL Cream, 1% even when the medicine is not on the skin. If a patient needs to be outdoors after applying ELIDEL Cream, 1%, the patient should wear loose fitting clothing that protects the treated area from the sun. The physician should advise the patient about other types of protection from the sun.
- A patient should not cover the skin being treated with bandages, dressings or wraps. A patient can wear normal clothing.
- ELIDEL Cream, 1% is for use on the skin only. Do not get ELIDEL Cream, 1% in your eyes, nose, mouth, vagina, or rectum (mucous membranes). If you get ELIDEL Cream, 1% in any of these areas, burning or irritation can happen. Wipe off any ELIDEL Cream, 1% from the affected area and then rinse the area well with cold water. ELIDEL Cream, 1% is for external use only.
- A patient should use ELIDEL Cream, 1% for short periods, and if needed, treatment may be repeated with breaks in between.
- Wash hands before using ELIDEL Cream, 1%. When applying ELIDEL Cream, 1% after a bath or shower, the skin should be dry.
- Apply a thin layer of ELIDEL Cream, 1% only to the affected skin areas, twice a day, as directed by the physician.
- Use the smallest amount of ELIDEL Cream, 1% needed to control the signs and symptoms of eczema.
- A patient should not bathe, shower or swim right after applying ELIDEL Cream, 1%. This could wash off the cream.
- A patient can use moisturizers with ELIDEL Cream, 1%. They should be sure to check with the physician first about the products that are right for them. Because the skin of patients with eczema can be very dry, it is important they keep up good skin care practices. If a patient uses moisturizers, he or she should apply them after ELIDEL Cream, 1%.
10MEDICATION GUIDE
ELIDEL
(pimecrolimus)
Cream, 1%
(pimecrolimus)
Cream, 1%
Important: ELIDEL Cream, 1% is for use on the skin only (topical). Do not get ELIDEL Cream, 1% in your eyes, nose, mouth, vagina, or rectum.
What is the most important information I should know about ELIDEL Cream, 1%?
It is not known if ELIDEL Cream, 1% is safe to use for a long period of time. A very small number of people who have used ELIDEL Cream, 1% have developed cancer (for example, skin cancer or lymphoma). But a link that ELIDEL Cream, 1% use caused these cancers has not been shown. Because of this concern:
- Do not use ELIDEL Cream, 1% continuously for a long time.
- Use ELIDEL Cream, 1% only on areas of your skin that have eczema.
- Do not use ELIDEL Cream, 1% on a child under 2 years of age.
What is ELIDEL Cream, 1%?
ELIDEL Cream, 1% is a prescription medicine used on the skin (topical) to treat mild to moderate eczema (atopic dermatitis). ELIDEL Cream, 1% is for adults and children age 2 years and older who do not have a weakened immune system. ELIDEL Cream, 1% is used on the skin for short periods, and if needed, treatment may be repeated with breaks in between. ELIDEL Cream, 1% is for use after other prescription medicines have not worked for you or if your doctor recommends that other prescription medicines should not be used.
It is not known if ELIDEL Cream, 1% is safe and effective in people who have a weakened immune system.
ELIDEL Cream, 1% is not for use in children under 2 years of age.
Who should not use ELIDEL Cream, 1%?
Do not use ELIDEL Cream, 1% if you are allergic to pimecrolimus or any of the ingredients in ELIDEL Cream, 1%. See the end of this Medication Guide for a complete list of ingredients in ELIDEL Cream, 1%.
What should I tell my doctor before using ELIDEL Cream, 1%?
Before using ELIDEL Cream, 1%, tell your doctor about all of your medical conditions, including if you:
- have a skin disease called Netherton’s syndrome (a rare inherited condition).
- have any infection on your skin including chickenpox or herpes.
- have been told you have a weakened immune system.
- are pregnant or plan to become pregnant. It is not known if ELIDEL Cream, 1% will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ELIDEL Cream, 1% passes into your breast milk. You and your doctor should decide if you will use ELIDEL Cream, 1% or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tell your doctor about all the skin medicines and products you use.
Know the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I use ELIDEL Cream, 1%?
- Use ELIDEL Cream, 1% exactly as your doctor tells you to use it.
- Stop ELIDEL Cream, 1% when the signs and symptoms of eczema, such as itching, rash, and redness go away, or as directed by your doctor.
- Wash your hands before using ELIDEL Cream, 1%. If you apply ELIDEL Cream, 1% after a bath or shower, make sure your skin is dry.
- Apply a thin layer of ELIDEL Cream, 1% only to the affected skin areas, two times each day, as directed by your doctor.
- Use the smallest amount of ELIDEL Cream, 1% to help control the signs and symptoms of eczema.
- If you apply ELIDEL Cream, 1% to another person, or if you have eczema and are not treating your hands, it is important for you to wash your hands with soap and water after applying ELIDEL Cream, 1%. This should remove any cream left on your hands.
- Do not bathe, shower or swim right after applying ELIDEL Cream, 1%. This could wash off the cream.
- You can use moisturizers with ELIDEL Cream, 1%. Ask your doctor first about the products that are right for you. People with eczema can have very dry skin, so it is important to keep up good skin care practices. If you use moisturizers, apply them after ELIDEL Cream, 1%.
- Call your doctor if your symptoms get worse with ELIDEL Cream, 1% or your symptoms do not improve after 6 weeks of treatment.
What should I avoid while using ELIDEL Cream, 1%?
- You should not use sun lamps, tanning beds, or get treatment with ultraviolet light therapy during treatment with ELIDEL Cream, 1%.
- Limit your time in the sun during treatment with ELIDEL Cream, 1% even when the medicine is not on your skin. If you need to be outdoors after applying ELIDEL Cream, 1%, wear loose fitting clothing that protects the treated area from the sun. Ask your doctor what other types of protection from the sun you should use. It is not known how ELIDEL Cream, 1% may affect your skin with exposure to ultraviolet light.
- Do not cover the skin being treated with bandages, dressings or wraps. You can wear normal clothing.
- ELIDEL Cream, 1% is for use on the skin only. Do not get ELIDEL Cream, 1% in your eyes, nose, mouth, vagina, or rectum (mucous membranes). If you get ELIDEL Cream, 1% in any of these areas, burning or irritation can happen. Wipe off any ELIDEL Cream, 1% from the affected area and then rinse the area well with cold water.
- Do not swallow ELIDEL Cream, 1%. If you do, call your doctor.
- Avoid using ELIDEL Cream, 1% on skin areas that have cancers or pre-cancers.
What are the possible side effects of ELIDEL Cream, 1%?
ELIDEL Cream, 1% may cause serious side effects.
- See “
- The most common side effect at the skin application site is burning or a feeling of warmth. These side effects are usually mild or moderate, happen during the first few days of treatment, and usually clear up in a few days.
Other common side effects include:
- headache
- common cold or stuffy nose, sore throat
- cough
- flu (influenza)
- fever
- viral infection. Some people may get viral skin infections (like cold sores, chickenpox, shingles, or warts) or swollen lymph nodes (glands).
Tell your doctor if you get a skin infection or if you have any side effect (for example, swollen glands) that bothers you or that does not go away.
These are not all the possible side effects with ELIDEL Cream, 1%. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ELIDEL Cream, 1%?
- Store ELIDEL Cream, 1% at room temperature between 68° to 77°F (20° to 25°C).
- Do not freeze ELIDEL Cream, 1%.
General information about the safe and effective use of ELIDEL Cream, 1%
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ELIDEL Cream, 1% for conditions other than which it was prescribed. Do not give ELIDEL Cream, 1% to other people even if they have the same symptoms you have. It may harm them.
You can ask your doctor or pharmacist for information about ELIDEL Cream, 1% that is written for health professionals.
For more information, call 1-800-321-4576.
What are the ingredients in ELIDEL Cream, 1%?
Active ingredient: pimecrolimus
Inactive ingredients: benzyl alcohol, cetyl alcohol, citric acid anhydrous, mono- and diglycerides, oleyl alcohol, propylene glycol, sodium cetostearyl sulphate, sodium hydroxide, stearyl alcohol, triglycerides and water
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
Produced under license from MEDA
ELIDEL is a trademark of MEDA PHARMA
© 2020 Bausch Health Companies Inc.
Rev. 09/2020 9462405
11PRINCIPAL DISPLAY PANEL - 100 g Carton
NDC 0187-5102-03
ELIDEL
(pimecrolimus)cream 1%
(pimecrolimus)cream 1%
Net Wt. 100 g
FOR TOPICAL USE ONLY.
NOT FOR OPHTHALMIC USE.
NOT FOR OPHTHALMIC USE.
If ELIDEL Cream gets in your eyes,
rinse your eyes with cold water.
rinse your eyes with cold water.
ATTENTION PHARMACIST:
Each patient is required to receive
the enclosed Medication Guide.
Each patient is required to receive
the enclosed Medication Guide.
Rx only

12PRINCIPAL DISPLAY PANEL - 60 g Carton
NDC 0187-5101-02
ELIDEL
(pimecrolimus)cream 1%
(pimecrolimus)cream 1%
Net Wt. 60 g
FOR TOPICAL USE ONLY.
NOT FOR OPHTHALMIC USE.
NOT FOR OPHTHALMIC USE.
If ELIDEL Cream gets in your eyes,
rinse your eyes with cold water.
rinse your eyes with cold water.
ATTENTION PHARMACIST:
Each patient is required to receive
the enclosed Medication Guide.
Each patient is required to receive
the enclosed Medication Guide.
Rx only

13PRINCIPAL DISPLAY PANEL - 30 g Carton
NDC 0187-5100-01
ELIDEL
(pimecrolimus)cream 1%
(pimecrolimus)cream 1%
Net Wt. 30 g
FOR TOPICAL USE ONLY.
NOT FOR OPHTHALMIC USE.
NOT FOR OPHTHALMIC USE.
If ELIDEL Cream gets in your eyes,
rinse your eyes with cold water.
rinse your eyes with cold water.
ATTENTION PHARMACIST:
Each patient is required to receive
the enclosed Medication Guide.
Each patient is required to receive
the enclosed Medication Guide.
Rx only

14Package/Label Display Panel
IMAGE NOT AVAILABLE




