Generic Name
Bumetanide
Brand Names
Bumex, Enbumyst
FDA approval date: February 28, 1983
Classification: Loop Diuretic
Form: Injection, Spray, Tablet
What is Bumex (Bumetanide)?
Bumetanide Injection is indicated for the treatment of edema associated with congestive heart failure, hepatic and renal disease, including the nephrotic syndrome. Almost equal diuretic response occurs after oral and parenteral administration of bumetanide. Therefore, if impaired gastrointestinal absorption is suspected or oral administration is not practical, bumetanide should be given by the intramuscular or intravenous route. Successful treatment with bumetanide following instances of allergic reactions to furosemide suggests a lack of cross-sensitivity.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Bumex (Bumetanide)
WARNING
Bumex
1DESCRIPTION
Bumex
Chemically, bumetanide is 3-(butylamino)-4-phenoxy-5-sulfamoylbenzoic acid. It is a practically white powder having a calculated molecular weight of 364.42, and the following structural formula:
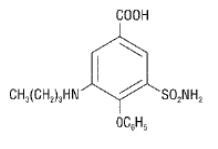
FDA-approved impurity specifications differ from the USP.
2CLINICAL PHARMACOLOGY
Bumex is a loop diuretic with a rapid onset and short duration of action. Pharmacological and clinical studies have shown that 1 mg Bumex has a diuretic potency equivalent to approximately 40 mg furosemide. The major site of Bumex action is the ascending limb of the loop of Henle.
The mode of action has been determined through various clearance studies in both humans and experimental animals. Bumex inhibits sodium reabsorption in the ascending limb of the loop of Henle, as shown by marked reduction of free-water clearance (CH
Potassium excretion is also increased by Bumex, in a dose-related fashion.
Bumex may have an additional action in the proximal tubule. Since phosphate reabsorption takes place largely in the proximal tubule, phosphaturia during Bumex induced diuresis is indicative of this additional action. This is further supported by the reduction in the renal clearance of Bumex by probenecid, associated with diminution in the natriuretic response. This proximal tubular activity does not seem to be related to an inhibition of carbonic anhydrase. Bumex does not appear to have a noticeable action on the distal tubule.
Bumex decreases uric acid excretion and increases serum uric acid. Following oral administration of Bumex the onset of diuresis occurs in 30 to 60 minutes. Peak activity is reached between 1 and 2 hours. At usual doses (1 mg to 2 mg) diuresis is largely complete within 4 hours; with higher doses, the diuretic action lasts for 4 to 6 hours. Diuresis starts within minutes following an intravenous injection and reaches maximum levels within 15 to 30 minutes.
Several pharmacokinetic studies have shown that bumetanide, administered orally or parenterally, is eliminated rapidly in humans, with a half-life of between 1 and 1½ hours. Plasma protein-binding is in the range of 94% to 96%.
Oral administration of carbon-14 labeled Bumex to human volunteers revealed that 81% of the administered radioactivity was excreted in the urine, 45% of it as unchanged drug. Urinary and biliary metabolites identified in this study were formed by oxidation of the N-butyl side chain. Biliary excretion of Bumex amounted to only 2% of the administered dose.
2.1Pediatric Pharmacology
Elimination of bumetanide appears to be considerably slower in neonatal patients compared with adults, possibly because of immature renal and hepatobiliary function in this population. Small pharmacokinetic studies of intravenous bumetanide in preterm and full-term neonates with respiratory disorders have reported an apparent half-life of approximately 6 hours, with a range up to 15 hours and a serum clearance ranging from 0.2 mL/min/kg to 1.1 mL/min/kg. In a population of neonates receiving bumetanide for volume overload, mean serum clearance rates were 2.2 mL/min/kg in patients less than 2 months of age and 3.8 mL/min/kg in patients aged 2 to 6 months. Mean serum half-life of bumetanide was 2.5 hours and 1.5 hours in patients aged less than 2 months and those aged 2 to 6 months, respectively. Elimination half-life decreased considerably during the first month of life, from a mean of approximately 6 hours at birth to approximately 2.4 hours at 1 month of age.
In preterm neonates, mean serum concentrations following a single 0.05 mg/kg dose ranged from 126 μg/L at 1 hour to 57 μg/L at 8 hours. In another study, mean serum concentrations following a single 0.05 mg/kg dose were 338 ng/mL at 30 minutes and 176 ng/mL after 4 hours. A single dose of 0.1 mg/kg produced mean serum levels of 314 ng/mL at 1 hour, and 195 ng/mL at 6 hours. Mean volume of distribution in neonates and infants has been reported to range from 0.26 L/kg to 0.39 L/kg.
The degree of protein binding of bumetanide in cord sera from healthy neonates was approximately 97%, suggesting the potential for bilirubin displacement. A study using pooled sera from critically ill neonates found that bumetanide at concentrations of 0.5 μg/mL to 50 μg/mL, but not 0.25 μg/mL, caused a linear increase in unbound bilirubin concentrations.
In 56 infants aged 4 days to 6 months, bumetanide doses ranging from 0.005 mg/kg to 0.1 mg/kg were studied for pharmacodynamic effect. Peak bumetanide excretion rates increased linearly with increasing doses of drug. Maximal diuretic effect was observed at a bumetanide excretion rate of about 7 μg/kg/h, corresponding to doses of 0.035 mg/kg to 0.040 mg/kg. Higher doses produced a higher bumetanide excretion rate but no increase in diuretic effect. Urine flow rate peaked during the first hour after drug administration in 80% of patients and by 3 hours in all patients.
2.2Geriatric Pharmacology
In a group of ten geriatric subjects between the ages of 65 and 73 years, total bumetanide clearance was significantly lower (1.8 ± 0.3 mL/min·kg) compared with younger subjects (2.9 ± 0.2 mL/min·kg) after a single oral bumetanide 0.5 mg dose. Maximum plasma concentrations were higher in geriatric subjects (16.9 ± 1.8 ng/mL) compared with younger subjects (10.3 ± 1.5 ng/mL). Urine flow rate and total excretion of sodium and potassium were increased less in the geriatric subjects compared with younger subjects, although potassium excretion and fractional sodium excretion were similar between the two age groups. Nonrenal clearance, bioavailability, and volume of distribution were not significantly different between the two groups.
3INDICATIONS AND USAGE
Bumex tablets are indicated for the treatment of edema associated with congestive heart failure, hepatic and renal disease, including the nephrotic syndrome.
Almost equal diuretic response occurs after oral and parenteral administration of bumetanide. Therefore, if impaired gastrointestinal absorption is suspected or oral administration is not practical, bumetanide should be given by the intramuscular or intravenous route.
Successful treatment with Bumex tablets following instances of allergic reactions to furosemide suggests a lack of cross-sensitivity.
4CONTRAINDICATIONS
Bumex is contraindicated in anuria. Although Bumex can be used to induce diuresis in renal insufficiency, any marked increase in blood urea nitrogen or creatinine, or the development of oliguria during therapy of patients with progressive renal disease, is an indication for discontinuation of treatment with Bumex. Bumex is also contraindicated in patients in hepatic coma or in states of severe electrolyte depletion until the condition is improved or corrected. Bumex is contraindicated in patients hypersensitive to this drug.
5ADVERSE REACTIONS
The most frequent clinical adverse reactions considered probably or possibly related to Bumex are muscle cramps (seen in 1.1% of treated patients), dizziness (1.1%), hypotension (0.8%), headache (0.6%), nausea (0.6%) and encephalopathy (in patients with pre-existing liver disease) (0.6%). One or more of these adverse reactions have been reported in approximately 4.1% of patients treated with Bumex.
Serious skin reactions (i.e., Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported in association with bumetanide use.
Less frequent clinical adverse reactions to Bumex are impaired hearing (0.5%), pruritus (0.4%), electrocardiogram changes (0.4%), weakness (0.2%), hives (0.2%), abdominal pain (0.2%), arthritic pain (0.2%), musculoskeletal pain (0.2%), rash (0.2%) and vomiting (0.2%). One or more of these adverse reactions have been reported in approximately 2.9% of patients treated with Bumex.
Other clinical adverse reactions, which have each occurred in approximately 0.1% of patients, are vertigo, chest pain, ear discomfort, fatigue, dehydration, sweating, hyperventilation, dry mouth, upset stomach, renal failure, asterixis, itching, nipple tenderness, diarrhea, premature ejaculation and difficulty maintaining an erection.
Laboratory abnormalities reported have included hyperuricemia (in 18.4% of patients tested), hypochloremia (14.9%), hypokalemia (14.7%), azotemia (10.6%), hyponatremia (9.2%), increased serum creatinine (7.4%), hyperglycemia (6.6%), and variations in phosphorus (4.5%), CO content (4.3%), bicarbonate (3.1%) and calcium (2.4%). Although manifestations of the pharmacologic action of Bumex, these conditions may become more pronounced by intensive therapy.
Also reported have been thrombocytopenia (0.2%) and deviations in hemoglobin (0.8%), prothrombin time (0.8%), hematocrit (0.6%), WBC (0.3%) and differential counts (0.1%). There have been rare spontaneous reports of thrombocytopenia from postmarketing experience.
Diuresis induced by Bumex may also rarely be accompanied by changes in LDH (1.0%), total serum bilirubin (0.8%), serum proteins (0.7%), SGOT (0.6%), SGPT (0.5%), alkaline phosphatase (0.4%), cholesterol (0.4%) and creatinine clearance (0.3%). Increases in urinary glucose (0.7%) and urinary protein (0.3%) have also been seen.
To report SUSPECTED ADVERSE REACTIONS, contact Validus Pharmaceuticals LLC at 1-866-982-5438 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6OVERDOSAGE
Overdosage can lead to acute profound water loss, volume and electrolyte depletion, dehydration, reduction of blood volume and circulatory collapse with a possibility of vascular thrombosis and embolism. Electrolyte depletion may be manifested by weakness, dizziness, mental confusion, anorexia, lethargy, vomiting and cramps. Treatment consists of replacement of fluid and electrolyte losses by careful monitoring of the urine and electrolyte output and serum electrolyte levels.
7DOSAGE AND ADMINISTRATION
Individualize dosage with careful monitoring of patient response.
7.1Oral Administration
The usual total daily dosage of Bumex tablets is 0.5 mg to 2 mg and in most patients is given as a single dose.
If the diuretic response to an initial dose of Bumex tablets is not adequate, in view of its rapid onset and short duration of action, a second or third dose may be given at 4- to 5-hour intervals up to a maximum daily dose of 10 mg. An intermittent dose schedule, whereby Bumex tablets are given on alternate days or for 3 to 4 days with rest periods of 1 to 2 days in between, is recommended as the safest and most effective method for the continued control of edema. In patients with hepatic failure, keep the dosage to a minimum.
Because cross-sensitivity with furosemide has rarely been observed, bumetanide can be substituted at approximately a 1:40 ratio of bumetanide in proportion to furosemide in patients allergic to furosemide.
7.2Parenteral Administration
Bumetanide injection may be administered parenterally (intravenously and intramuscularly) to patients in whom gastrointestinal absorption may be impaired or in whom oral administration is not practical.
Terminate parenteral treatment and institute oral treatment as soon as possible.
8HOW SUPPLIED
Bumex Tablets for oral administration are elliptical, flat-faced, and bevel-edged, available as:
Store at 68° to 77°F (20° to 25°C); excursions permitted between 59° to 86°F (15° to 30°C) [See USP Controlled Room Temperature].
Dispense contents in a tight, light-resistant container as defined in the USP with a child-resistant closure, as required.
Manufactured for andDistributed by:
Validus Pharmaceuticals LLC
Parsippany, NJ 07054
info@validuspharma.com
www.validuspharma.com
1-866-982-5438
Validus Pharmaceuticals LLC
Parsippany, NJ 07054
info@validuspharma.com
www.validuspharma.com
1-866-982-5438
Product of Italy
© 2023 Validus Pharmaceuticals LLC
60018-05 November 2023
9PRINCIPAL DISPLAY PANEL
NDC 30698-630-01

10PRINCIPAL DISPLAY PANEL
NDC 30698-631-01

NDC 30698-631-05

11PRINCIPAL DISPLAY PANEL
NDC 30698-632-01

NDC 30698-632-05
