Generic Name
Selegiline
Brand Names
EMSAM, Zelapar
FDA approval date: August 02, 1996
Classification: Monoamine Oxidase Inhibitor
Form: Patch, Tablet, Capsule
What is EMSAM (Selegiline)?
Selegiline capsules, USP are indicated as an adjunct in the management of Parkinsonian patients being treated with levodopa/carbidopa who exhibit deterioration in the quality of their response to this therapy. There is no evidence from controlled studies that selegiline has any beneficial effect in the absence of concurrent levodopa therapy. Evidence supporting this claim was obtained in randomized controlled clinical investigations that compared the effects of added selegiline or placebo in patients receiving levodopa/carbidopa. Selegiline was significantly superior to placebo on all three principal outcome measures employed: change from baseline in daily levodopa/carbidopa dose, the amount of 'off' time, and patient self-rating of treatment success. Beneficial effects were also observed on other measures of treatment success .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
EMSAM (selegiline)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors
EMSAM is contraindicated in patients less than 12 years of age because of an increased risk of hypertensive crisis
1INDICATIONS AND USAGE
EMSAM (selegiline transdermal system) is a monoamine oxidase inhibitor (MAOI) indicated for the treatment of adults with major depressive disorder (MDD)
2DOSAGE FORMS AND STRENGTHS
EMSAM (selegiline transdermal system) is supplied as 6 mg per 24 hours (20 mg per 20 cm
EMSAM
3CONTRAINDICATIONS
- EMSAM (selegiline transdermal system) is contraindicated with selective serotonin reuptake inhibitors (SSRIs, e.g., fluoxetine, sertraline, and paroxetine); serotonin and norepinephrine reuptake inhibitors (SNRIs, e.g., venlafaxine and duloxetine); the tricyclic antidepressants clomipramine and imipramine, the opiate analgesics meperidine, tramadol, methadone, pentazocine, and propoxyphene; and the antitussive agent dextromethorphan because of a risk of serotonin syndrome when EMSAM is used with these agents
- Carbamazepine is contraindicated with EMSAM because of a possible increased risk of hypertensive crisis
- After stopping treatment with drugs contraindicated with EMSAM, a time period equal to 4 to 5 half-lives (approximately one week) of the drug or any active metabolite should elapse before starting therapy with EMSAM. Because of the long half-life of fluoxetine and its active metabolite, at least 5 weeks should elapse between discontinuation of fluoxetine and initiation of treatment with EMSAM.
- At least 2 weeks should elapse after stopping EMSAM before starting therapy with any drug that is contraindicated with EMSAM.
- EMSAM is contraindicated in patients less than 12 years of age because of the potential for a hypertensive crisis
- EMSAM is contraindicated in patients with pheochromocytoma because MAOIs may precipitate a hypertensive crisis in such patients.
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label.
- Suicidal Thoughts and Behaviors
- Serotonin Syndrome
- Blood Pressure Elevation
- Activation of Mania/Hypomania
- External Heat
4.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
4.1.1Patient Exposure
The premarketing development program for EMSAM included selegiline exposures in patients and/or normal subjects from two different groups of studies: 702 healthy subjects in clinical pharmacology/pharmacokinetics studies and 2,036 exposures from patients in controlled and uncontrolled major depressive disorder clinical trials. The conditions and duration of treatment with EMSAM varied and included double-blind, open-label, fixed-dose, and dose titration studies of short-term and longer-term exposures. Safety was assessed by monitoring adverse reactions, physical examinations, vital signs, body weights, laboratory analyses, and ECGs.
Adverse reactions during exposure were obtained primarily by general inquiry and recorded by clinical investigators. In the tables and tabulations that follow, standard COSTART terminology has been used to classify reported adverse reactions. The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse reaction of the type listed. A reaction was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
4.1.2Adverse Reactions Leading To Discontinuation of Treatment
Among 817 MDD patients treated with EMSAM at doses of either 3 mg per 24 hours (151 patients), 6 mg per 24 hours (550 patients) or 6 mg per 24 hours, 9 mg per 24 hours, and 12 mg per 24 hours (116 patients) in placebo-controlled trials of up to 8 weeks in duration, 7.1% discontinued treatment due to an adverse reaction as compared with 3.6% of 668 patients receiving placebo. The only adverse reaction associated with discontinuation, in at least 1% of EMSAM-treated patients at a rate at least twice that of placebo, was application site reaction (2% EMSAM vs. 0% placebo).
4.1.3Adverse Reactions Occurring at an Incidence of 2% or More Among EMSAM-Treated Patients
Table 2 enumerates adverse reactions that occurred at an incidence of 2% or more (rounded to the nearest percent) among 817 MDD patients treated with EMSAM in doses ranging from 3 to 12 mg per 24 hours in placebo-controlled trials of up to 8 weeks in duration. Reactions included are those occurring in 2% or more of patients treated with EMSAM and for which the incidence in patients treated with EMSAM was greater than the incidence in placebo-treated patients.
One adverse reaction was associated with a reporting of at least 5% in the EMSAM group, and a rate at least twice that in the placebo group, in the pool of short-term, placebo-controlled studies: application site reactions (
4.1.4Application Site Reactions
In the pool of short-term, placebo-controlled major depressive disorder studies, application site reactions (ASRs) were reported in 24% of EMSAM-treated patients and 12% of placebo-treated patients. Most ASRs were mild or moderate in severity. ASRs led to dropout in 2% of EMSAM-treated patients and no placebo-treated patients. In one such study which utilized higher mean doses of EMSAM, ASRs were reported in 40% of EMSAM-treated patients and 20% of placebo-treated patients. Most of the ASRs in this study were described as erythema and most resolved spontaneously, requiring no treatment. When treatment was administered, it most commonly consisted of dermatological preparations of corticosteroids.
4.1.5Sexual Dysfunction
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment.
Reliable estimates of the incidence and severity of untoward experiences involving sexual desire, performance, and satisfaction are difficult to obtain, in part because patients and physicians may be reluctant to discuss them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling are likely to underestimate their actual incidence. Table 3 shows that the incidence rates of sexual side effects in patients with major depressive disorder are comparable to the placebo rates in placebo-controlled trials.
There are no adequately designed studies examining sexual dysfunction with EMSAM treatment.
4.1.6Vital Sign Changes
EMSAM and placebo groups were compared with respect to (1) mean change from baseline in vital signs (pulse, systolic blood pressure, and diastolic blood pressure), and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. In the pool of short-term, placebo-controlled major depressive disorder studies, 3.0% of EMSAM-treated patients and 1.5% of placebo-treated patients experienced a low systolic blood pressure, defined as a reading less than or equal to 90 mmHg with a change from baseline of at least 20 mmHg. In one study which utilized higher mean doses of EMSAM, 6.2% of EMSAM-treated patients and no placebo-treated patients experienced a low standing systolic blood pressure by these criteria.
In the pool of short-term major depressive disorder trials, 9.8% of EMSAM-treated patients and 6.7% of placebo-treated patients experienced a notable orthostatic change in blood pressure, defined as a decrease of at least 10 mmHg in mean blood pressure with postural change.
4.1.7Weight Changes
In placebo-controlled studies (6 to 8 weeks), the incidence of patients who experienced at least 5% weight gain or weight loss is shown in Table 4.
In these trials, the mean change in body weight among EMSAM-treated patients was a 1.2 lbs loss compared to 0.3 lbs gain in placebo-treated patients.
4.1.8Laboratory Changes
EMSAM and placebo groups were compared with respect to (1) mean change from baseline in various serum chemistry, hematology, and urinalysis variables, and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses revealed no clinically important changes in laboratory test parameters associated with EMSAM.
4.1.9Electrocardiogram Changes
Electrocardiograms (ECGs) from EMSAM (N = 817) and placebo (N = 668) groups in controlled studies were compared with respect to (1) mean change from baseline in various ECG parameters, and (2) the incidence of patients meeting criteria for clinically significant changes from baseline in these variables.
No clinically meaningful changes in ECG parameters from baseline to final visit were observed for patients in controlled studies.
4.1.10Other Reactions Observed During the Premarketing Evaluation of EMSAM
The following listing does not include reactions: 1) already listed elsewhere in labeling, 2) for which a causal relationship to drug was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo.
Cardiovascular System:Tachycardia.
Digestive System:Anorexia.
Nervous System:Agitation, amnesia, tremor, twitching.
Skin and Appendages:Pruritus.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of EMSAM.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous System: Convulsion and hypoesthesia.
Psychiatric System: Disorientation, hallucination (visual), and tension.
5DRUG ABUSE AND DEPENDENCE
EMSAM is not a controlled substance.
6DESCRIPTION
EMSAM
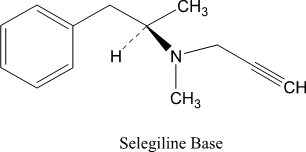
Selegiline base is a colorless to yellow liquid, chemically described as (-)-(
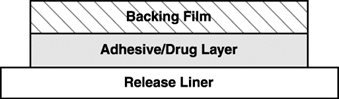
EMSAM transdermal systems are available in three strengths that deliver approximately 6 mg, 9 mg, or 12 mg of selegiline over 24 hours. Each corresponding system has an active surface area of 20 cm
EMSAM is a matrix-type transdermal system composed of three layers as illustrated in Figure 1 below. Layer 1 is the Backing Film that provides occlusivity, physical integrity and protects the adhesive/drug layer. Layer 2 is the Adhesive/Drug Layer. Layer 3 consists of side-by-side release liners that are peeled off and discarded by the patient prior to applying EMSAM. The inactive ingredients are acrylic adhesive, ethylene vinyl acetate/polyethylene, polyester, polyurethane, and silicone coated polyester.
7REFERENCES
- Adapted from K.I. Shulman, S.E. Walker, Psychiatric Annals 2001; 31:378-384
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
EMSAM (selegiline transdermal system) is a transdermal system with the following strengths, sizes, color, backing film printing and presentation:
Storage and Handling
Store at 20° to 25° C (68° to 77° F). [See USP Controlled Room Temperature.] Do not store outside of the sealed pouch.
Apply immediately upon removal from the protective pouch. Discard used EMSAM in household trash in a manner that prevents accidental application or ingestion by children, pets or others.
9PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (
Advise patients and their caregivers about the benefits and risks associated with treatment with EMSAM and counsel them in its appropriate use. Advise patients and their caregivers to read the Medication Guide and assist them in understanding its contents. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking EMSAM.
Suicide Risk: Advise patients and caregivers to look for the emergence of suicidal ideation and behavior, especially early during treatment and when the dose is adjusted up or down [see .
Tyramine Reactions: Patients should be advised that tyramine-rich foods and beverages should be avoided while on EMSAM 9 mg per 24 hours or EMSAM 12 mg per 24 hours, and for 2 weeks following discontinuation of EMSAM at these doses because of the risk of a tyramine reaction [see . Patients should also be advised to avoid tyramine-containing nutritional supplements. Patients should be instructed to immediately report the occurrence of the following acute symptoms: severe headache, neck stiffness, heart racing or palpitations, or other sudden or unusual symptoms.
Concomitant Medication: Advise patients to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter medications, including herbals, because of a potential for dangerous interactions. Instruct patients not to take EMSAM with medication that is contraindicated or within two weeks of stopping such medication (5 weeks for fluoxetine). Contraindicated medication should not be started within two weeks of stopping EMSAM [see .
Psychomotor Performance: EMSAM has not been shown to impair psychomotor performance; however, any psychoactive drug may potentially impair judgment, thinking, or motor skills. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that EMSAM therapy does not impair their ability to engage in such activities.
Alcohol: Patients should be told that, although EMSAM has not been shown to increase the impairment of mental and motor skills caused by alcohol, the concomitant use of EMSAM and alcohol in depressed patients is not recommended.
Pediatrics: Advise patients that EMSAM must not be used in children less than 12 years of age because of an increased risk of severe increases in blood pressure. Also, patients should be advised that EMSAM is not recommended for use in pediatric patients ages 12 to 17 years [see .
Pregnancy: Advise the pregnant woman about the potential risk to the fetus [see
Lactation: Advise a woman that breastfeeding is not recommended during treatment with EMSAM treatment and for 5 days after the final dose [see .
How to Use EMSAM
Detailed Instructions are provided in the Medication Guide. Prescribers should instruct patients on the following:
- EMSAM should be applied to dry, intact skin on the upper torso (below the neck and above the waist), upper thigh or the outer surface of the upper arm. A new application site should be selected with each new transdermal system to
- Apply the transdermal system to an area of skin that is not hairy, oily, irritated, broken, scarred or calloused. Do not place the transdermal system where your clothing is tight, which could cause the transdermal system to rub off.
- After you have selected the site for your transdermal system, wash the area gently and thoroughly with soap and warm water. Rinse until all soap is removed. Dry the area with a clean dry towel.
- Just before you apply the transdermal system, remove it from the pouch by tearing at the notches (do not use scissors). Remove half of the release liner and throw it away. Try not to touch the exposed side (sticky side) of the transdermal system, because the medicine could come off on your fingers.
- Press the sticky side of the transdermal system firmly against the skin site that was just washed and dried. Remove the second half of the release liner and press the remaining sticky side firmly against your skin. Make sure that the transdermal system is flat against the skin (there should be no bumps or folds in the transdermal system) and is sticking securely. Be sure the edges are stuck to the skin surface.
- After you have applied the transdermal system,
- After 24 hours, remove your transdermal system slowly and carefully to avoid damaging your skin.
- If the transdermal system is too sticky on your skin, and you need something to help you remove it:
- Fold the used EMSAM transdermal system in half and press it together firmly so that the sticky side sticks to itself.
- Safely throw away the folded transdermal system in a container with a lid right away so that children and pets cannot reach it.
- Safely throw away any unused EMSAM transdermal systems that are left over from the prescription as soon as they are no longer needed.
- Wash your hands with soap and water.
- If your transdermal system falls off, apply a new transdermal system to a new site and resume your previous schedule.
- Only one EMSAM transdermal system should be worn at a time.
- Do not cut the EMSAM transdermal system into smaller portions.
The brands listed are trademarks of their respective owners.
10MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration
11Instructions for Use
EMSAM
(selegiline transdermal system)
(selegiline transdermal system)
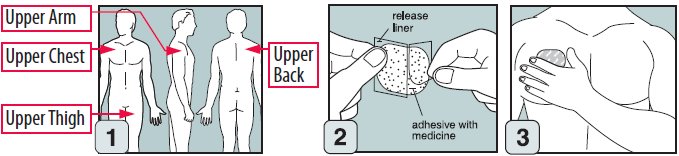
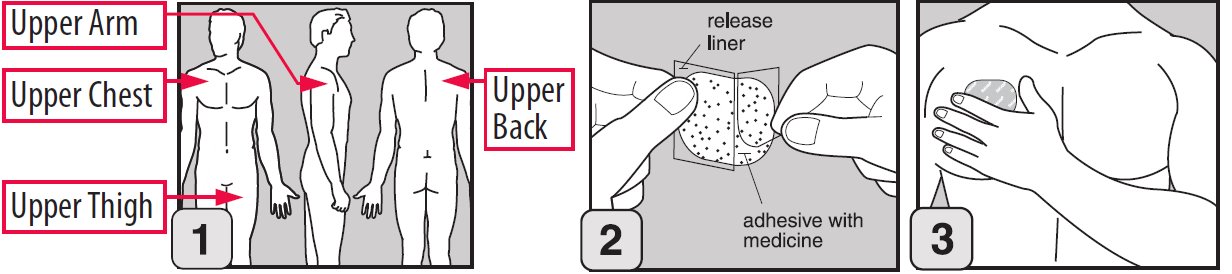
Step 1. Where to apply EMSAM
- Place your EMSAM transdermal system (patch) on one of the following areas (sites) on your body.
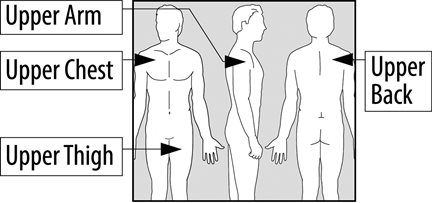
- EMSAM should be applied to dry, intact skin on the upper torso (below the neck and above the waist), upper thigh or the outer surface of the upper arm. Clothing and movement may make your patch rub off.
- Choose a new site each time you change your patch. Do not use the same site 2 days in a row.
Step 2. Before you apply EMSAM
- Make sure the area on your skin where you apply your patch:
Step 3. How to apply EMSAM
- Remove EMSAM from its sealed pouch by tearing at the notches (do not use scissors). Pull the pouch open.
- Look at the patch to make sure it is not damaged. The patch should separate easily from the release liner. Throw away the patch if the release liner is hard to remove.
- Do not keep or store your EMSAM outside of the sealed pouch. Do not cut your EMSAM into smaller pieces.
- The EMSAM patch has three layers.
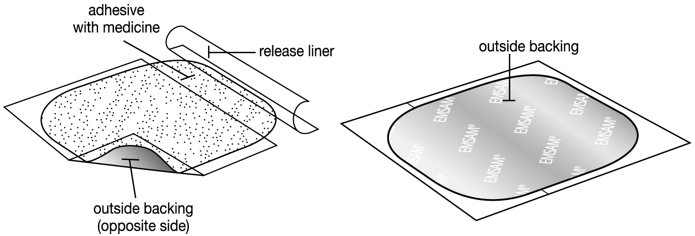
- Layers:
- Release liner: The release liner is the layer that you remove before you put the patch on. See Figure C.
- Adhesive with medicine: The adhesive with medicine is the layer that sticks to your skin. See Figure C.
- Outside backing: The outside backing is the layer you see after you put the patch on your skin. See Figure D.
- Apply the patch right away after you remove the patch from its sealed pouch.
- Hold the patch with the release liner facing you.
- Gently peel half of the release liner off the patch and throw it away.
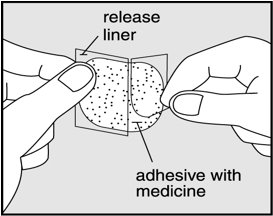
- Avoid touching the sticky side of the patch with your fingers. If you accidently touch the sticky side of the patch, wash your hands right away so the medicine does not go into the skin on your hands.
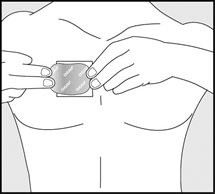
- Using the other half of the release liner as a handle, apply the sticky side of the patch to your selected area.
- Hold an edge of the remaining half of the release liner and slowly peel it off.
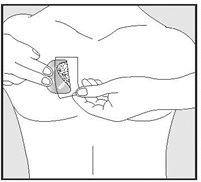
- After the release liner is removed, there should not be any adhesive sticking to the liner.
- Using your fingers and palm of your hand, press the entire patch firmly into place against your skin.
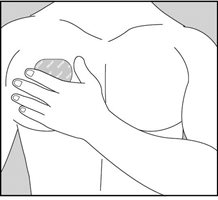
- Make sure the patch firmly sticks to your skin.
- Gently rub the edges of your patch with your fingers to make sure the patch sticks to your skin.
- Wash your hands well with soap and water after you apply your patch to remove any medicine.
- If the patch becomes loose, press it back in place. If your EMSAM patch falls off, apply a new EMSAM patch to a new site and follow your normal schedule for changing patches.
- If you forget to change your patch after 24 hours, remove the old patch. Put on a new patch in a different area and continue to follow your normal schedule for changing patches.
Step 4. Removing and disposing of your patch
- After 24 hours, remove your patch slowly and carefully to avoid damaging your skin.
- If the patch is too sticky on your skin and you need something to help you remove it:
- Fold the used EMSAM patch in half and press it together firmly so that the sticky side sticks to itself.
- Safely throw away the folded patch in a container with a lid right away so that children and pets cannot reach it.
- Safely throw away any unused EMSAM patches that are left over from the prescription as soon as they are no longer needed.
- To safely throw away the patches:
- Wash your hands with soap and water.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
DISTRIBUTED BY:
MANUFACTURED FOR:
Revised: 5/2020
12PRINCIPAL DISPLAY PANEL - 6 mg/24 h
NDC-49502-900-30
EACH UNIT DOSE PACKAGE IS NOT CHILD RESISTANT
Rx only
Rx only
EMSAM
(selegiline transdermal system)
6 mg/24 h
(selegiline transdermal system)
6 mg/24 h
FOR TRANSDERMAL USE ONLY
Patient: Read enclosed Medication Guide.
Pharmacist: Dispense with enclosed
Medication Guide.
Medication Guide.
30 TRANSDERMAL
SYSTEMS
SYSTEMS
INSTRUCTIONS FOR APPLICATION
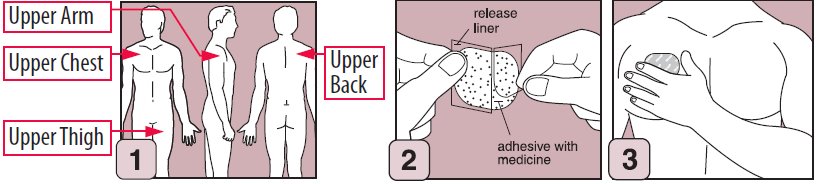
1. Select and prepare an appropriate site on the upper chest or back (below the neck and above the waist), the upper thigh, or the outer surface of the upper arm, as directed in the enclosed Medication Guide.
2. Bend both sides of the clear peelable liner. Peel off one strip only of the clear liner. Avoid touching the sticky side of the transdermal system.
3. Apply sticky side of the transdermal system to the chosen skin site. Remove remaining strip, gently roll remaining part onto skin, and press transdermal system firmly in place with the tips of your fingers.
For more detailed instructions, read enclosed Medication Guide.
Dosage: Apply one (1) transdermal system every 24 hours. Read enclosed Medication Guide.
Each transdermal system has a release rate of 6 mg selegiline per 24 hour period. Wear only one transdermal system at a time.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dist. by
Mfd. for
Rx only
S033:93:30C:R10
13PRINCIPAL DISPLAY PANEL - 9 mg/24 h
NDC-49502-901-30
EACH UNIT DOSE PACKAGE IS NOT CHILD RESISTANT
Rx only
Rx only
EMSAM
(selegiline transdermal system)
9 mg/24 h
(selegiline transdermal system)
9 mg/24 h
Important: Do not eat certain foods.
Read enclosed Medication Guide.
Read enclosed Medication Guide.
FOR TRANSDERMAL USE ONLY
Pharmacist: Dispense with enclosed
Medication Guide.
Medication Guide.
30 TRANSDERMAL
SYSTEMS
SYSTEMS
INSTRUCTIONS FOR APPLICATION
1. Select and prepare an appropriate site on the upper chest or back (below the neck and above the waist), the upper thigh, or the outer surface of the upper arm, as directed in the enclosed Medication Guide.
2. Bend both sides of the clear peelable liner. Peel off one strip only of the clear liner. Avoid touching the sticky side of the transdermal system.
3. Apply sticky side of the transdermal system to the chosen skin site. Remove remaining strip, gently roll remaining part onto skin, and press transdermal system firmly in place with the tips of your fingers.
For more detailed instructions, read enclosed Medication Guide.
Dosage: Apply one (1) transdermal system every 24 hours. Read enclosed Medication Guide.
Each transdermal system has a release rate of 9 mg selegiline per 24 hour period. Wear only one transdermal system at a time.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dist. by
Mfd. for
Rx only
S044:93:30C:R10

14PRINCIPAL DISPLAY PANEL - 12 mg/24 h
NDC-49502-902-30
EACH UNIT DOSE PACKAGE IS NOT CHILD RESISTANT
Rx only
Rx only
EMSAM
(selegiline transdermal system)
12 mg/24 h
(selegiline transdermal system)
12 mg/24 h
Important: Do not eat certain foods.
Read enclosed Medication Guide.
Read enclosed Medication Guide.
FOR TRANSDERMAL USE ONLY
Pharmacist: Dispense with enclosed
Medication Guide.
Medication Guide.
30 TRANSDERMAL
SYSTEMS
SYSTEMS
INSTRUCTIONS FOR APPLICATION
1. Select and prepare an appropriate site on the upper chest or back (below the neck and above the waist), the upper thigh, or the outer surface of the upper arm, as directed in the enclosed Medication Guide.
2. Bend both sides of the clear peelable liner. Peel off one strip only of the clear liner. Avoid touching the sticky side of the transdermal system.
3. Apply sticky side of the transdermal system to the chosen skin site. Remove remaining strip, gently roll remaining part onto skin, and press transdermal system firmly in place with the tips of your fingers.
For more detailed instructions, read enclosed Medication Guide.
Dosage: Apply one (1) transdermal system every 24 hours. Read enclosed Medication Guide.
Each transdermal system has a release rate of 12 mg selegiline per 24 hour period. Wear only one transdermal system at a time.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dist. by
Mfd. for
Rx only
S055:93:30C:R10
