Generic Name
Aspart
Brand Names
NovoLog, Remodulin, Yutrepia, Treprostinil, Orenitram, Tyvaso DPI, Tyvaso, Merilog, FiAsp
FDA approval date: August 27, 2001
Classification: Prostacycline Vasodilator
Form: Injection, Inhalant, Tablet, Kit, Capsule
What is NovoLog (Aspart)?
Treprostinil injection is a prostacyclin mimetic indicated for: Treatment of pulmonary arterial hypertension to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH , PAH associated with congenital systemic-to-pulmonary shunts , or PAH associated with connective tissue diseases .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
NOVOLOG (insulin aspart)
1INDICATIONS AND USAGE
NOVOLOG is indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
2DOSAGE FORMS AND STRENGTHS
Injection: 100 units/mL (U-100) is a clear and colorless solution available as:
- 10 mL multiple-dose vial
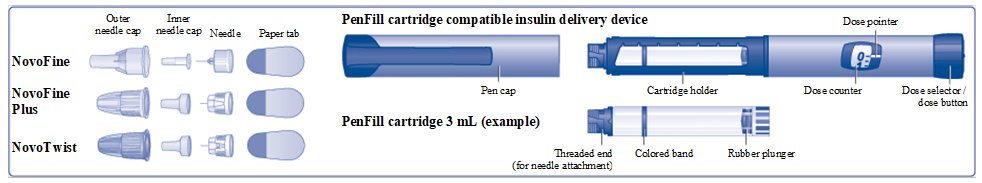
- 3 mL single-patient-use PenFill prefilled cartridge for the 3 mL PenFill cartridge delivery device with NovoFine
- 3 mL single-patient-use FlexPen prefilled pen
- 3 mL single-patient-use FlexTouch prefilled pen
3CONTRAINDICATIONS
NOVOLOG is contraindicated:
- During episodes of hypoglycemia
- In patients with hypersensitivity to NOVOLOG or one of its excipients,
4ADVERSE REACTIONS
- The following adverse reactions are also discussed elsewhere:
- Hypoglycemia
- Hypoglycemia Due to Medication Errors
- Hypersensitivity reactions
- Hypokalemia
4.1Clinical Trial Experience
Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice. The safety of NOVOLOG was evaluated in two treat-to-target trials of 6 months duration, conducted in patients with type 1 diabetes or type 2 diabetes
The data in Table 1 reflect the exposure of 596 patients with type 1 diabetes to NOVOLOG in one clinical trial with a mean exposure duration to NOVOLOG of 24 weeks. The mean age was 39 years. Fifty-one percent were male, 94% were Caucasian, 2% were Black and 4% were other races. The mean body mass index (BMI) was 25.6 kg/m
The data in Table 2 reflect the exposure of 91 patients with type 2 diabetes to NOVOLOG in one clinical trial with a mean exposure duration to NOVOLOG of 24 weeks. The mean age was 57 years. Sixty-three percent were male, 76% were Caucasian, 9% were Black and 15% were other races. The mean BMI was 29.7 kg/m
Common adverse reactions were defined as events that occurred in ≥5%, excluding hypoglycemia, of the population studied. Common adverse events that occurred at the same rate or greater for NOVOLOG-treated patients than in comparator-treated patients during clinical trials in patients with type 1 diabetes mellitus and type 2 diabetes mellitus (other than hypoglycemia) are listed in Table 1 and Table 2, respectively.
Table 1: Adverse reactions that occurred in ≥ 5% of Type 1 Diabetes Mellitus Adult Patients treated with NOVOLOG and at the same rate or greater on NOVOLOG than on comparator
Table 2: Adverse reactions that occurred in ≥ 5% of Type 2 Diabetes Mellitus Adult Patients treated with NOVOLOG and at the same rate or greater on NOVOLOG than on comparator
Severe Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including NOVOLOG
Severe hypoglycemia was defined as hypoglycemia associated with central nervous system symptoms and requiring the intervention of another person or hospitalization. The incidence of severe hypoglycemia in:
- Adult and pediatric patients with type 1 diabetes mellitus who received subcutaneous NOVOLOG was 17% at 24 weeks and 6% at 24 weeks, respectively
- Adult patients with type 2 diabetes mellitus who received subcutaneous NOVOLOG was 10% at 24 weeks.
- Adult and pediatric patients with type 1 diabetes mellitus, who received NOVOLOG via continuous subcutaneous insulin infusion by external pump was 2% at 16 weeks and 10% at 16 weeks respectively.
No severe hypoglycemic episodes were reported in adult patients with type 2 diabetes mellitus receiving NOVOLOG via continuous subcutaneous insulin infusion by external pump at 16 weeks.
Allergic Reactions
Some patients taking insulin, including NOVOLOG have experienced erythema, local edema, and pruritus at the site of injection. These conditions were usually self-limiting. Severe cases of generalized allergy (anaphylaxis) have been reported
Adverse Reactions Associated with Insulin Initiation and Glucose Control Intensification
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. However, long-term glycemic control decreases the risk of diabetic retinopathy and neuropathy.
Lipodystrophy
Administration of insulin, including NOVOLOG, subcutaneously and via subcutaneous insulin infusion by external pump, has resulted in lipoatrophy (depression in the skin) or lipohypertrophy (enlargement or thickening of tissue) in some patients
Peripheral Edema
Insulins, including NOVOLOG, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Weight Gain
Weight gain has occurred with insulins, including NOVOLOG, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria.
4.2Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to NOVOLOG in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In a 6-month study with a 6-month extension in adult subjects with type 1 diabetes, 99.8% of patients who received NOVOLOG were positive for anti-insulin antibodies (AIA) at least once during the study, including 97.2% that were positive at baseline. A total of 92.1% of patients who received NOVOLOG were positive for anti-drug antibodies (ADA) at least once during the study, including 64.6% that were positive at baseline.
In a phase 3 type 1 diabetes clinical trial of NOVOLOG, initial increase in titers of antibodies to insulin, followed by a decrease to baseline values, was observed in regular human insulin and insulin aspart treatment groups with similar incidences. These antibodies did not cause deterioration in glycemic control or necessitate increases in insulin dose.
4.3Post Marketing Experience
The following adverse reactions have been identified during post-approval use of NOVOLOG. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Medication errors have been reported in which other insulins have been accidentally substituted for NOVOLOG.
Localized cutaneous amyloidosis at the injection site has occurred with insulin aspart. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
5DRUG INTERACTIONS
The table below presents clinically significant drug interactions with NOVOLOG.
6OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia
7DESCRIPTION
Insulin aspart is a rapid-acting human insulin analog homologous with regular human insulin with the exception of a single substitution of the amino acid proline by aspartic acid in position B28, and is produced by recombinant DNA technology utilizing
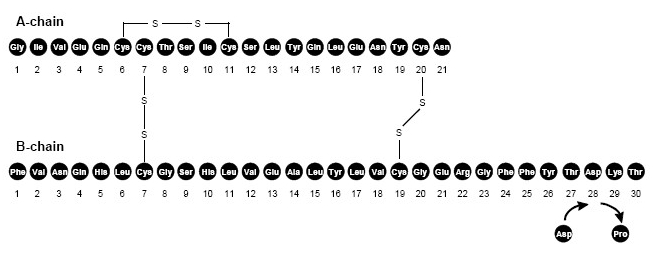
NOVOLOG (insulin aspart) injection is a sterile, clear, and colorless solution for subcutaneous or intravenous use. Each mL contains 100 units of insulin aspart and the inactive ingredients: disodium hydrogen phosphate dihydrate (1.25 mg), glycerin (16.0 mg), metacresol (1.72 mg), phenol (1.50 mg), sodium chloride (0.58 mg), zinc (19.6 mcg), and Water for Injection, USP. NOVOLOG has a pH of 7.2-7.6. Hydrochloric acid 10% and/or sodium hydroxide 10% may be added to adjust pH.
8HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-4500
NDC: 50090-4500-0 3 mL in a SYRINGE, PLASTIC / 5 in a CARTON
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Never Share a NOVOLOG FlexPen or a NOVOLOG FlexTouch, PenFill Cartridge or PenFill Cartridge Device between Patients
Advise patients that they must never share NOVOLOG FlexPen, NOVOLOG FlexTouch, PenFill cartridge or PenFill cartridge devices with another person even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens. Advise patients using NOVOLOG vials not to share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens
Hyperglycemia or Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Instruct patients on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia, especially at initiation of NOVOLOG therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals. Instruct patients on the management of hypoglycemia
Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery.
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision
Hypoglycemia with Medication Errors
Instruct patients to always check the insulin label before each injection to avoid mix-ups between insulin products
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with NOVOLOG. Inform patients of the symptoms of hypersensitivity reactions
Patients Using Continuous Subcutaneous Insulin Pumps
- Train patients in both intensive insulin therapy with multiple injections and in the function of their pump and pump accessories.
- This NOVOLOG product can be used with continuous subcutaneous insulin infusion pumps labeled for use with NOVOLOG (insulin aspart) - refer to the insulin pump user manual to see if NOVOLOG can be used. See recommended infusion sets in the insulin pump user manual.
- Instruct patients to replace insulin in the reservoir at least every 7 days or according to the user manual, whichever is shorter; infusion sets and infusion set insertion sites should be changed according to the manufacturer’s user manual. By following this schedule, patients avoid insulin degradation, infusion set occlusion, and loss of the insulin preservative.
- Instruct patients to discard insulin exposed to temperatures higher than 37°C (98.6°F).
- Instruct patients to inform physician and select a new site for infusion if infusion site becomes erythematous, pruritic, or thickened.
- Instruct patients of the risk of rapid hyperglycemia and ketosis due to pump malfunction, infusion set occlusion, leakage, disconnection or kinking, and degraded insulin. If these problems cannot be promptly corrected, instruct patients to resume therapy with subcutaneous insulin injection and contact their physician
- Instruct patients of the risk of hypoglycemia from pump malfunction. If these problems cannot be promptly corrected, instruct patients to resume therapy with subcutaneous insulin injection and contact their physician
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
Version: 30
Novo Nordisk
ReliOn
Patent Information: http://novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
For additional information about NOVOLOG contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500 (Se Habla español)
10PATIENT INFORMATION
NovoLog
(insulin aspart)
injection, for subcutaneous or intravenous use
Do not share your NovoLog FlexPen, NovoLog FlexTouch, PenFill cartridge or PenFill cartridge compatible insulin delivery device with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
What is NovoLog?
- NovoLog is a man-made insulin that is used to control high blood sugar in adults and children with diabetes mellitus.
Who should not take NovoLog?
Do not take NovoLog if you:
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to NovoLog or any of the ingredients in NovoLog.
Before taking NovoLog, tell your healthcare provider about all your medical conditions including, if you are:
- pregnant, planning to become pregnant, or are breastfeeding.
- taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
Before you start taking NovoLog, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take NovoLog?
- Read the Instructions for Use that come with your NovoLog.
- Take NovoLog exactly as your healthcare provider tells you to.
- NovoLog starts acting fast. You should eat a meal within 5 to 10 minutes after you take your dose of NovoLog.
- Know the type and strength of insulin you take.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
- Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
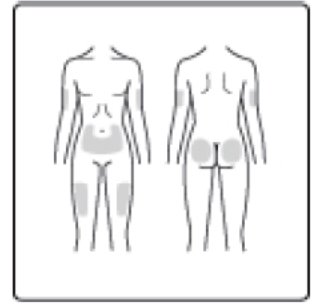
- NovoLog can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs) or upper arms, or by continuous infusion under the skin (subcutaneously) through an insulin pump into an area of your body recommended in the instructions that come with your insulin pump.
- Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
What should I avoid while taking NovoLog?
While taking NovoLog do not:
- Drive or operate heavy machinery, until you know how NovoLog affects you.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of NovoLog?
NovoLog may cause serious side effects that can lead to death, including:
Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- dizziness or light-headedness
- blurred vision
- anxiety, irritability, or mood changes
- sweating
- slurred speech
- hunger
- confusion
- shakiness
- headache
- fast heart beat
Your insulin dose may need to change because of:
- change in level of physical activity or exercise
- increased stress
- change in diet
- weight gain or loss
- illness
Other common side effects of NovoLog may include:
- low potassium in your blood (hypokalemia), reactions at the injection site, itching, rash, serious allergic reactions (whole body reactions), skin thickening or pits at the injection site (lipodystrophy), weight gain, and swelling of your hands and feet.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, swelling of your face, tongue, or throat, sweating, extreme drowsiness, dizziness, confusion.
These are not all the possible side effects of NovoLog. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of NovoLog.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about NovoLog that is written for health professionals. Do not use NovoLog for a condition for which it was not prescribed. Do not give NovoLog to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in NovoLog?
Active Ingredient: insulin aspart
Inactive Ingredients: disodium hydrogen phosphate dihydrate, glycerin, metacresol, phenol, sodium chloride, zinc, and Water for Injection, USP. Hydrochloric acid 10% and/or sodium hydroxide 10% may be added to adjust pH.
Manufactured by: Novo Nordisk Inc., Plainsboro, NJ 08536 U.S. License Number 1261
For more information, go to www.novonordisk-us.com or call 1-800-727-6500.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 02/2023
INSTRUCTIONS FOR USE
NovoLog
(insulin aspart)
injection, for subcutaneous or intravenous use
10 mL multiple-dose vial (100 Units/mL, U-100)
Read this Instructions for Use before you start taking NovoLog and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Supplies you will need to give your NovoLog injection:
- 10 mL NovoLog vial
- insulin syringe and needle
- alcohol swabs
Preparing your NovoLog dose:
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the NovoLog label to make sure that you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- NovoLog should look clear and colorless.
- Do not use NovoLog past the expiration date printed on the label.
Step 1: Pull off the tamper resistant cap (See Figure A).
Step 2: Wipe the rubber stopper with an alcohol swab (See Figure B).
Step 3: Hold the syringe with the needle pointing up. Pull down on the plunger until the black tip reaches the line for the number of units for your prescribed dose (See Figure C).
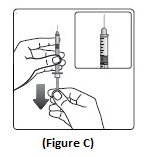
Step 4: Push the needle through the rubber stopper of the NovoLog vial (See Figure D).
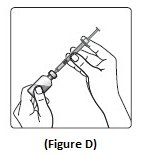
Step 5: Push the plunger all the way in. This puts air into the NovoLog vial (See Figure E).
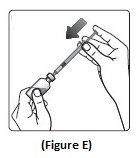
Step 6: Turn the NovoLog vial and syringe upside down and slowly pull the plunger down until the black tip is a few units past the line for your dose (See Figure F).
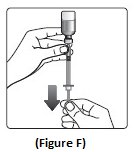
- If there are air bubbles, tap the syringe gently a few times to let any air bubbles rise to the top (See Figure G).
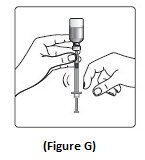
Step 7: Slowly push the plunger up until the black tip reaches the line for your NovoLog dose (See Figure H).
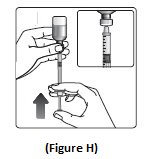
Step 8: Check the syringe to make sure you have the right dose of NovoLog.
Step 9: Pull the syringe out of the vial’s rubber stopper (See Figure I).
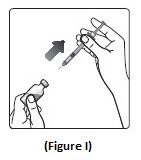
- Giving your Injection:
- Inject your NovoLog exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- NovoLog can be injected under the skin (subcutaneously) of your stomach area, buttocks, upper legs or upper arms, infused in an insulin pump (continuous subcutaneous infusion into an area of your body recommended in the instructions that come with your insulin pump), or given through a needle in your arm (intravenously) by your healthcare provider.
- If you inject NovoLog,
- If you use NovoLog in an insulin pump, you should change your infusion set and insertion site according to the manufacturer’s user manual. NovoLog should be given into an area of your body recommended in the instructions that come with your insulin pump. Change (rotate) your insertion sites within the area you choose for each insertion to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the insertion sites. Do not insert into the exact same spot for each insertion. Do not insert where the skin has pits, is thickened, or has lumps. Do not insert where the skin is tender, bruised, scaly or hard, or into scars or damaged skin. The insulin in the reservoir should be changed at least every 7 days or according to the pump user manual, whichever is shorter, even if you have not used all of the insulin.
- If you use NovoLog in an insulin pump, see your insulin pump manual for instructions or talk to your healthcare provider.
- NPH insulin is the only type of insulin that can be mixed with NovoLog.
- NovoLog should
- NovoLog should be drawn up into the syringe
- Talk to your healthcare provider if you are not sure about the right way to mix NovoLog and NPH insulin.
Step 10: Choose your injection site (stomach area, buttocks, upper legs or upper arms) and wipe the skin with an alcohol swab. Let the injection site dry before you inject your dose (See Figure J).
(Figure J)
Step 11: Insert the needle into your skin. Push down on the plunger to inject your dose (See Figure K). The needle should remain in the skin for at least 6 seconds to make sure you have injected all the insulin.
Step 12: Pull the needle out of your skin. After that, you may see a drop of NovoLog at the needle tip. This is normal and does not affect the dose you just received (See Figure L).
- If you see blood after you take the needle out of your skin, press the injection site lightly with a
- piece of gauze or an alcohol swab.
After your injection:
- Do not recap the needle. Recapping the needle can lead to a needle stick injury.
- Put the empty insulin vials, used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes and needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
How should I store NovoLog?
- Do not freeze NovoLog. Do not use NovoLog if it has been frozen.
- Keep NovoLog away from heat or light.
- All unopened vials:
- Store unopened NovoLog vials in the refrigerator at 36
- Unopened vials may be used until the expiration date printed on the label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 28 days, if they are stored at room temperature.
- After vials have been opened:
- Opened NovoLog vials can be stored in the refrigerator at 36
- Throw away all opened NovoLog vials after 28 days, even if they still have insulin left in them.
- If using NovoLog in a pump, throw away all opened NovoLog vials after 19 days.
General information about the safe and effective use of NovoLog
- Always use a new syringe and needle for each injection.
- Do not share syringes or needles.
- Keep NovoLog vials, syringes, and needles out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
U.S. License Number 1261
NovoLog.
Patent Information:
© 2023 Novo Nordisk
For information about NovoLog contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
www.novonordisk-us.com
Revised: 02/2023
INSTRUCTIONS FOR USE
NovoLog® (NŌ-vō-log)
(insulin aspart)
injection, for subcutaneous or intravenous use
PenFill
- Do not share your PenFill cartridge or PenFill cartridge compatible insulin delivery device with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- Your healthcare provider should show you or your caregiver how to inject NovoLog the right way before you inject it for the first time.
- NovoLog PenFill cartridge 100 units/mL is a prefilled, single-patient-use cartridge containing 300 units of NovoLog (insulin aspart).
- After you insert the PenFill cartridge in your device, you can use it for multiple injections. Read the
- People who are blind or have vision problems should not use this PenFill cartridge without help from a person trained to use the PenFill cartridge with the device.
- If using a
- If the NovoLog PenFill cartridge has already been
Supplies you will need to give your NovoLog injection:
- NovoLog PenFill cartridge
- Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device
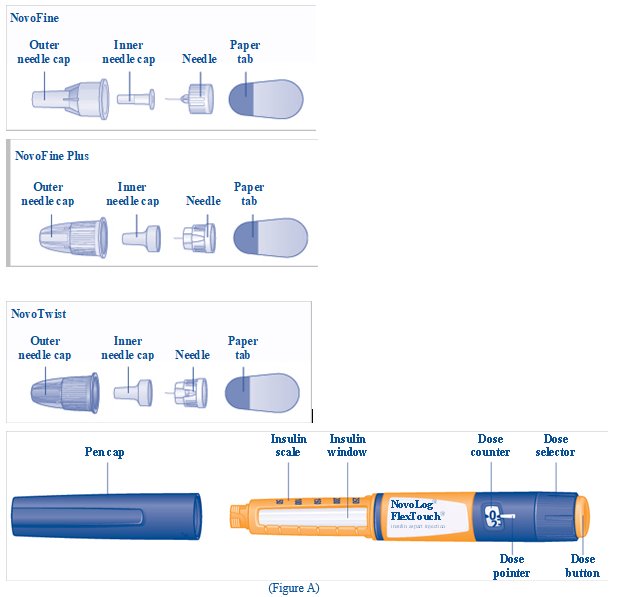
- 1 new NovoFine, NovoFine Plus, or NovoTwist needle
- Alcohol swabs
- Adhesive bandage
- Cotton gauze
- A sharps container for throwing away used PenFill cartridges and needles.
(Figure A)
How to use the NovoLog PenFill cartridge
- Wash your hands with soap and water.
- Before you start to prepare your injection,
- The tamper-resistant foil should be in place before the first use. If the foil has been broken or removed before your first use of the cartridge,
- Carefully look at the cartridge and the insulin inside it. Check that the NovoLog cartridge:
- NovoLog should look clear and colorless.
(Figure B)
Step 1:
- Insert a 3 mL cartridge with the threaded end first into your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device (See Figure C).
- If you drop your device, check the insulin cartridge for damage such as cracks or leaking. If your cartridge is damaged, throw it away and use a new one.
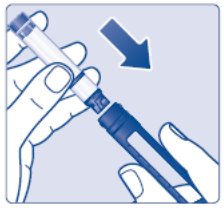
(Figure C)
Prepare your device with a new needle
Step 2:
- Take
- Be careful not to bend or damage the needle before you use it.
- Push the needle straight onto the device. Turn the needle clockwise until it is on tight (See Figure D).
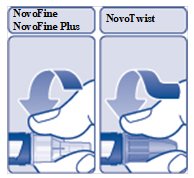
(Figure D)
Step 3:
- Pull off the outer needle cap (See Figure E). Do not throw it away. You will need it after the injection to safely remove the needle.
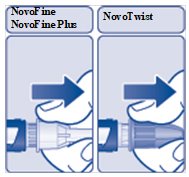
(Figure E)
Step 4:
- Pull off the inner needle cap and throw it away (See Figure F). Do not try to put the inner needle cap back on the needle.
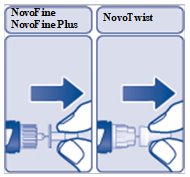
(Figure F)
A drop of insulin may appear at the needle tip. This is normal, but you must still check the insulin flow.
- Check the insulin flow
Step 5:
- Small amounts of air may collect in the cartridge during normal use. You must do an airshot before each injection to avoid injecting air and to make sure you receive the prescribed dose of your medicine.
- Do the airshot as described in the instruction manual that comes with your device.
- Keep testing your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device until you see insulin at the needle tip. If you still do not see a drop of insulin after 6 times, change the needle and repeat this step. This makes sure that any air bubbles are removed and that insulin is getting through the needle (See Figure G).
(Figure G)
Select your dose
Step 6:
- Check to make sure that the dose counter is set to 0.
- Turn the dose selector clockwise to select the dose you need to inject (See Figure H). The pointer should line up with your dose. When turning the dose selector, be careful not to press the dose button as insulin will come out. You will hear a click for every single unit dialed. Do not set the dose by counting the number of clicks you hear because you may get an incorrect dose.
- Refer to your insulin delivery device manual if necessary.
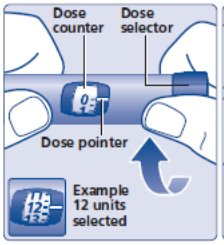
(Figure H)
Inject your dose
Step 7:
- Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- NovoLog can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs), or upper arms (See Figure I).
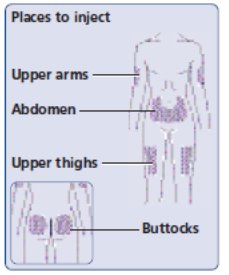
- (Figure I)
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Clean your injection site with an alcohol swab. Let your skin dry. Do not touch this area again before injecting.
- Insert the needle into your skin. Press and hold down the dose button until the dose counter shows “0”. Continue to keep the dose button pressed and keep the needle in your skin and slowly count to 6 (see Figure J).
- Remove the needle from your skin.
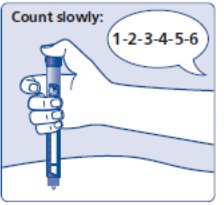
(Figure J)
You may see a drop of NovoLog at the needle tip after injecting. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with a cotton gauze and cover with an adhesive bandage, if necessary.
After your injection
Step 8:
- Lay your outer needle cap on a flat surface. Carefully, lead the needle tip into the outer needle cap without touching the needle (See Figure K) and push the outer needle cap completely on.
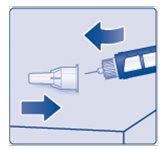
(Figure K)
- Hold the black cartridge holder on the insulin delivery device and unscrew the needle counterclockwise (See Figure L).
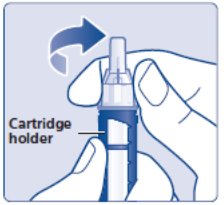
(Figure L)
- Throw away (dispose of) the needle in an FDA-cleared sharps container as your healthcare professional has instructed you.
- Put your empty NovoLog PenFill cartridge and used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and PenFill cartridges in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Step 9:
- Keep the 3 mL PenFill cartridge in the device.
- Put the pen cap on your device after each use to protect the insulin from light (See Figure M).
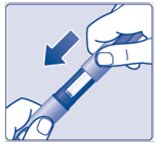
(Figure M)
How should I store my NovoLog PenFill cartridge?
- Do not freeze NovoLog. Do not use NovoLog if it has been frozen.
- Keep NovoLog away from heat or light.
- Store the NovoLog PenFill cartridge without the needle attached.
Before use:
- Store unused NovoLog PenFill cartridges in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused PenFill cartridges may be used until the expiration date printed on the label, if kept in the refrigerator.
- If NovoLog is stored mistakenly outside of refrigeration between 47°F (9°C) to 86°F (30°C) prior to first use, it should be used within 28 days or thrown away.
PenFill cartridges in use:
- Store the PenFill cartridge you are currently using in the insulin delivery device at room temperature below 86°F (30°C) for up to 28 days. Do not refrigerate.
- The NovoLog PenFill cartridge you are using should be thrown away after 28 days, even if it still has insulin left in it.
General Information about the safe and effective use of NovoLog.
- Keep NovoLog PenFill cartridges and needles out of the reach of children.
- Do not share NovoLog PenFill cartridges or needles with other people. You may give other people a serious infection, or get a serious infection from them.
- Always carry extra insulin of the same type(s) you use in case of loss or damage.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2023 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
Revised: 02/2023
INSTRUCTIONS FOR USE
NovoLog
(insulin aspart)
injection, for subcutaneous or intravenous use
3 mL FlexTouch
- Do not share your NovoLog FlexTouch Pen with other people, even if the needle has been is changed. You may give other people a serious infection, or get a serious infection from them.
- NovoLog FlexTouch Pen (“Pen”) is a prefilled, single-patient-use disposable pen containing 300 units of U-100 NovoLog (insulin aspart injection) insulin. You can inject from 1 to 80 units in a single injection. The units can be increased by 1 unit at a time.
- People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.
Supplies you will need to give your NovoLog injection:
- NovoLog FlexTouch Pen
- a new NovoFine, NovoFine Plus or NovoTwist needle
- alcohol swabs
- 1 sharps container for throwing away used Pens and needles.
Preparing your NovoLog FlexTouch Pen:
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the NovoLog FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- NovoLog should look clear and colorless.
- Do not use NovoLog past the expiration date printed on the label or 28 days after you start using the Pen.
Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.
Step 1:
- Pull Pen cap straight off (See Figure B).
(Figure B)
Step 2:
- Check the liquid in the Pen (See Figure C). NovoLog should look clear and colorless. Do not use it if it looks cloudy or colored.
(Figure C)
Step 3:
- Select a new needle.
- Pull off the paper tab from the outer needle cap (See Figure D).
(Figure D)
Step 4:
- Push the capped needle straight onto the Pen and twist the needle on until it is tight (See Figure E).
(Figure E)
Step 5:
- Pull off the outer needle cap.
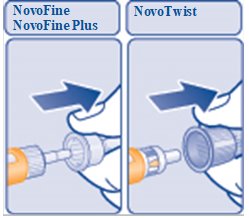
(Figure F)
Step 6:
- Pull off the inner needle cap and throw it away (See Figure G).
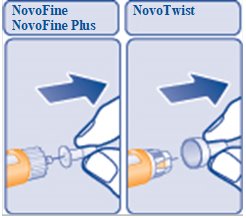
(Figure G)
Priming your NovoLog FlexTouch Pen:
Step 7:
- Turn the dose selector to
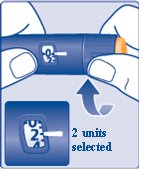
(Figure H)
Step 8:
- Hold the Pen with the needle pointing up. Tap the top of the Pen gently a few times to let any air bubbles rise to the top (See Figure I).
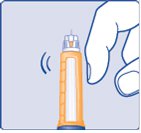
(Figure I)
Step 9:
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter shows “0”. The “0” must line up with the dose pointer.
- A drop of insulin should be seen at the needle tip (See Figure J).
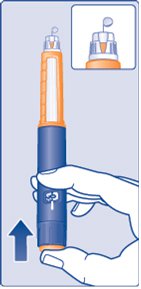
(Figure J)
Selecting your dose:
Step 10:
- Turn the dose selector to select the number of units you need to inject. The dose pointer should line up with your dose (See Figure K).
- If you select the wrong dose, you can turn the dose selector forwards or backwards to the correct dose.
- The
- The
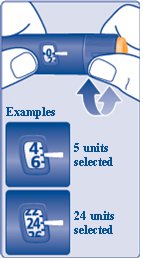
(Figure K)
- The NovoLog FlexTouch Pen insulin scale will show you how much insulin is left in your Pen (See Figure L).

(Figure L)
- To see how much insulin is left in your NovoLog FlexTouch Pen:
- Turn the dose selector until it stops. The dose counter will line up with the number of units of insulin that is left in your Pen. If the dose counter shows 80, there are
- If the dose counter shows
Giving your injection:
- Inject your NovoLog exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- NovoLog can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs) or upper arms.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. For each injection, change (rotate) your injection site within the area of skin that you use.
Step 11:
- Choose your injection site and wipe the skin with an alcohol swab. Let the injection site dry before you inject your dose (See Figure M).
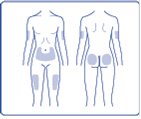
(Figure M)
Step 12:
- Insert the needle into your skin (See Figure N).
- Make sure you can see the dose counter. Do not cover it with your fingers, this can stop your injection.
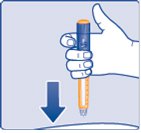
(Figure N)
Step 13:
- Press and hold down the dose button until the dose counter shows “0” (See Figure O).
- The “0” must line up with the dose pointer. You may then hear or feel a click.

(Figure O)
- Keep the needle in your skin after the dose counter has returned to “0” and slowly count to 6 (See Figure P).

(Figure P)
- When the dose counter returns to “0”, you will not get your full dose until 6 seconds later.
- If the needle is removed before you count to 6, you may see a stream of insulin coming from the needle tip.
- If you see a stream of insulin coming from the needle tip you will not get your full dose. If this happens you should check your blood sugar levels more often because you may need more insulin.
Step 14:
- Pull the needle out of your skin (See Figure Q).
- If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol swab.
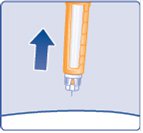
(Figure Q)
Step 15:
- Carefully remove the needle from the Pen and throw it away (See Figure R).
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
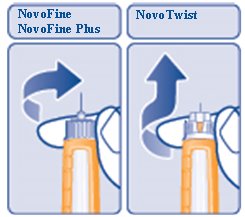
(Figure R)
- If you
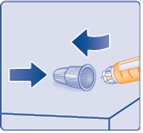
(Figure S)
- Do not store the Pen with the needle attached. Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
Step 16:
- Replace the Pen cap by pushing it straight on (See Figure T).
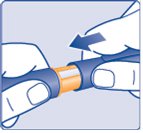
(Figure T)
After your injection:
- You can put your used NovoLog FlexTouch Pen and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and Pens in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my NovoLog FlexTouch Pen?
- Do not freeze NovoLog. Do not use NovoLog if it has been frozen.
- Keep NovoLog away from heat or light.
- Store the NovoLog FlexTouch Pen without the needle attached.
- Until first use:
- Store unused NovoLog FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused NovoLog FlexTouch Pen stored at room temperature should be thrown away after 28 days.
- In-use:
- Store the Pen you are currently using out of the refrigerator at room temperature below 86
- The NovoLog FlexTouch Pen you are using should be thrown away after 28 days, even if it still has insulin left in it.
General Information about the safe and effective use of NovoLog.
- Keep NovoLog FlexTouch Pens and needles out of the reach of children.
- Always use a new needle for each injection.
- Do not share your NovoLog FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261

Revised: 02/2023
For more information go to
© 2023 Novo Nordisk

INSTRUCTIONS FOR USE
NovoLog
(insulin aspart)
Injection, for subcutaneous or intravenous use
3mL FlexPen
Introduction
Please read the following instructions carefully before using your NovoLog FlexPen.
Do not share your NovoLog FlexPen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
NovoLog FlexPen is a disposable, single-patient-use, dial-a-dose insulin pen. You can select doses from 1 to 60 units in increments of 1 unit. NovoLog FlexPen is designed to be used with NovoFine, NovoFine Plus or NovoTwist needles.
- Δ People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.
Getting ready
Make sure you have the following items:
- NovoLog FlexPen
- New NovoFine, NovoFine
- Alcohol swabs
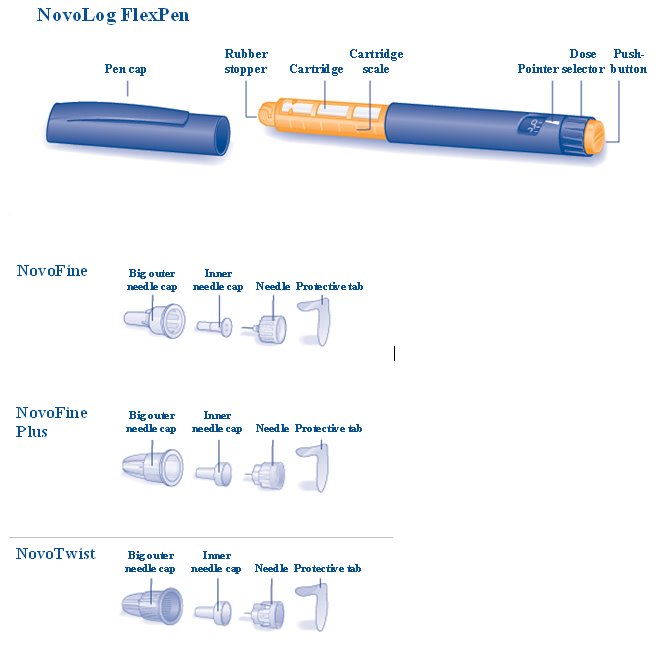
Preparing your NovoLog FlexPen
Wash your hands with soap and water. Before you start to prepare your injection, check the label to make sure that you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin. NovoLog should look clear and colorless. Do not use your NovoLog FlexPen if the liquid contains particles or is colored.
A. Pull off the pen cap (see diagram A).

Wipe the rubber stopper with an alcohol swab.
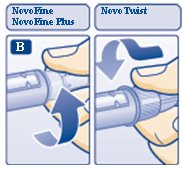
B. Attaching the needle
Remove the protective tab from a disposable needle.
Screw the needle tightly onto your FlexPen. It is important that the needle is put on straight (see diagram B).
Never place a disposable needle on your NovoLog FlexPen until you are ready to take your injection.
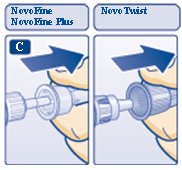
C. Pull off the big outer needle cap (see diagram C).
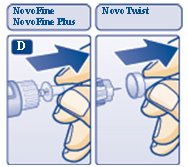
D. Pull off the inner needle cap and throw it away (dispose of it) (see diagram D).
- Δ Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.
- Δ Be careful not to bend or damage the needle before use.
- Δ To reduce the risk of unexpected needle sticks, never put the inner needle cap back on the needle.
Giving the airshot before each injection
Before each injection small amounts of air may collect in the cartridge during normal use. To avoid injecting air and to ensure proper dosing:
E. Turn the dose selector to select 2 units (see diagram E).

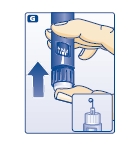
F. Hold your NovoLog FlexPen with the needle pointing up. Tap the cartridge gently with your finger a few times to make any air bubbles collect at the top of the cartridge (see diagram F).
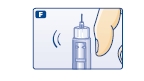
G. Keep the needle pointing upwards, press the push-button all the way in (see diagram G). The dose selector returns to 0.
A drop of insulin should appear at the needle tip. If not, change the needle and repeat the procedure no more than 6 times.
If you do not see a drop of insulin after 6 times, do not use the NovoLog FlexPen and contact Novo Nordisk at 1-800-727-6500.
A small air bubble may remain at the needle tip, but it will not be injected.
Selecting your dose
Check and make sure that the dose selector is set at 0.
H. Turn the dose selector to the number of units you need to inject. The pointer should line up with your dose.
The dose can be corrected either up or down by turning the dose selector in either direction until the correct dose lines up with the pointer (see diagram H). When turning the dose selector, be careful not to press the push-button as insulin will come out.
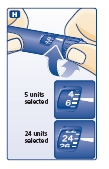
You cannot select a dose larger than the number of units left in the cartridge.
You will hear a click for every single unit dialed. Do not set the dose by counting the number of clicks you hear because you may get an incorrect dose.
- Δ Do not use the cartridge scale printed on the cartridge to measure your dose of insulin.
Giving the injection
Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting. Wipe the skin with an alcohol swab and let the area dry.
NovoLog can be injected under the skin (subcutaneously) of your stomach area, buttocks, upper legs (thighs), or upper arms.
Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
I. Insert the needle into your skin.
Inject the dose by pressing the push-button all the way in until the 0 lines up with the pointer (see diagram I). Be careful only to push the button when injecting.
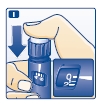
Turning the dose selector will not inject insulin.
J. Keep the needle in the skin for at least 6 seconds, and keep the push-button pressed all the way in until the needle has been pulled out from the skin (see diagram J). This will make sure that the full dose has been given.
You may see a drop of insulin at the needle tip. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with an alcohol swab.
After the injection
Do not recap the needle. Recapping can lead to a needle stick injury. Remove the needle from the NovoLog FlexPen after each injection and dispose of it. This helps to prevent infection, leakage of insulin, and will help to make sure you inject the right dose of insulin.
If you do not have a sharps container, carefully slip the needle into the outer needle cap. Safely remove the needle and throw it away as soon as you can.
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- When there is not enough medicine left in your NovoLog FlexPen for your prescribed dose, the NovoLog FlexPen may be thrown away in your household trash after you have removed the needle. The NovoLog FlexPen prevents the cartridge from being completely emptied. It is designed to deliver 300 units.
K. Put the pen cap on the NovoLog FlexPen and store the NovoLog FlexPen without the needle attached (see diagram K).
Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
How should I store NovoLog FlexPen?
- Do not freeze NovoLog. Do not use NovoLog if it has been frozen.
- Keep NovoLog away from heat or light.
- Store the NovoLog FlexPen without the needle attached.
- Until first use:
- Store unused NovoLog FlexPen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused FlexPen may be used until the expiration date printed on the label, if kept in the refrigerator.
- Unused NovoLog FlexPen stored at room temperature should be thrown away after 28 days.
- In-use:
- Store the FlexPen you are currently using out of the refrigerator at room temperature below 86°F (30°C) for up to 28 days.
- The NovoLog FlexPen you are using should be thrown away after 28 days, even if it still has insulin left in it.
Maintenance
For the safe and proper use of your FlexPen be sure to handle it with care. Avoid dropping your FlexPen as it may damage it. If you are concerned that your FlexPen is damaged, use a new one. You can clean the outside of your FlexPen by wiping it with a damp cloth. Do not soak or wash your FlexPen as it may damage it. Do not refill your FlexPen.
Δ Remove the needle from the NovoLog FlexPen after each injection. This helps to ensure sterility, prevent leakage of insulin, and will help to make sure you inject the right dose of insulin for future injections.
Δ Be careful when handling used needles to avoid needle sticks and transfer of infectious diseases.
Δ Keep your NovoLog FlexPen and needles out of the reach of children.
Δ Use NovoLog FlexPen as directed to treat your diabetes.
Δ Do not share your NovoLog FlexPen or needles with other people. You may give other people a serious infection, or
get a serious infection from them.
Δ Always use a new needle for each injection.
Δ Novo Nordisk is not responsible for harm due to using this insulin pen with products not recommended by Novo
Nordisk.
Δ As a precautionary measure, always carry a spare insulin delivery device in case your NovoLog FlexPen is lost or damaged.
Δ Remember to keep the disposable NovoLog FlexPen with you. Do not leave it in a car or other location where it can get too hot or too cold.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
U.S. License Number 1261
Revised: 02/2023
11insulin aspart
