Generic Name
Lacosamide
Brand Names
Motpoly, Vimpat
FDA approval date: May 26, 2009
Form: Injection, Tablet, Capsule, Solution
What is Motpoly (Lacosamide)?
Partial-Onset Seizures Lacosamide is indicated for the treatment of partial-onset seizures in patients 1 month of age and older. Lacosamide is indicated for: Treatment of partial-onset seizures in patients 1 month of age and older. Primary Generalized Tonic-Clonic Seizures Lacosamide is indicated as adjunctive therapy in the treatment of primary generalized tonic-clonic seizures in patients 4 years of age and older.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
MOTPOLY XR (lacosamide)
1CONTRAINDICATIONS
None
2ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Suicidal Behavior and Ideation
- Dizziness and Ataxia
- Cardiac Rhythm and Conduction Abnormalities
- Syncope
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Reactions
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
2.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of lacosamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Agranulocytosis
3OVERDOSAGE
Events reported after an intake of more than 800 mg (twice the maximum recommended daily dosage) of lacosamide include dizziness, nausea, and seizures (generalized tonic-clonic seizures, status epilepticus). Cardiac conduction disorders, confusion, decreased level of consciousness, cardiogenic shock, cardiac arrest, and coma have also been observed. Fatalities have occurred following lacosamide overdoses of several grams.
There is no specific antidote for overdose with lacosamide. Standard decontamination procedures should be followed. General supportive care of the patient is indicated including monitoring of vital signs and observation of the clinical status of patient. A Certified Poison Control Center should be contacted for up to date information on the management of overdose with lacosamide.
Standard hemodialysis procedures result in significant clearance of lacosamide (reduction of systemic exposure by 50% in 4 hours). Hemodialysis may be indicated based on the patient's clinical state or in patients with significant renal impairment.
4DESCRIPTION
The chemical name of lacosamide, the single (R)-enantiomer, is (R)-2-acetamido-N-benzyl-3-methoxypropionamide (IUPAC). Lacosamide is a functionalized amino acid. Its molecular formula is C
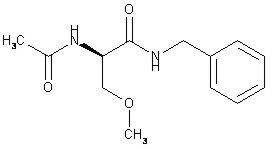
Lacosamide is a white to light yellow powder. It is sparingly soluble in water and slightly soluble in acetonitrile and ethanol.
5PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide).
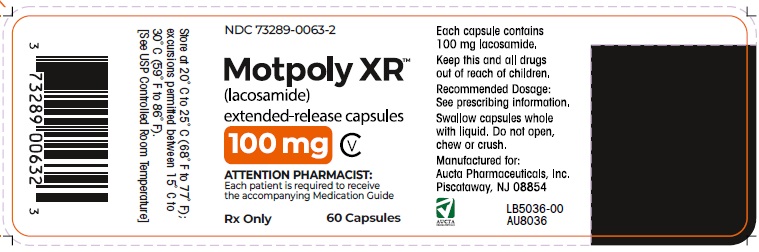
6PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
NDC 73289-0063-2
Motpoly XR (lacosamide) extended-release capsules
CV
CV
100 mg
Rx only 60 Capsules
ATTENTION PHARMACIST:
Each patient is required to receive
the accompanying Medication Guide
Each patient is required to receive
the accompanying Medication Guide
Each capsule contains
Keep this and all drugs
Recommended Dosage:
Swallow capsules whole
Manufactured for:
Store at 20˚ C to 25˚ C (68˚ F to 77˚ F);
7PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
NDC 73289-0064-2
Motpoly XR (lacosamide) extended-release capsules
150 mg
Rx only
ATTENTION PHARMACIST:
Each patient is required to receive the
accompanying Medication Guide.
Each patient is required to receive the
accompanying Medication Guide.
Each capsule contains
Keep this and all drugs
Recommended Dosage:
Swallow capsules whole
Manufactured for:
Store at 20˚ C to 25˚ C (68˚ F to 77˚ F);

8PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle Label
NDC 73289-0065-2
Motpoly XR (lacosamide) extended-release capsules
200 mg
Rx only
ATTENTION PHARMACIST:
Each patient is required to receive the
accompanying Medication Guide.
Each patient is required to receive the
accompanying Medication Guide.
Each capsule contains
Keep this and all drugs
Recommended Dosage:
Swallow capsules whole
Manufactured for:
Store at 20˚ C to 25˚ C (68˚ F to 77˚ F);

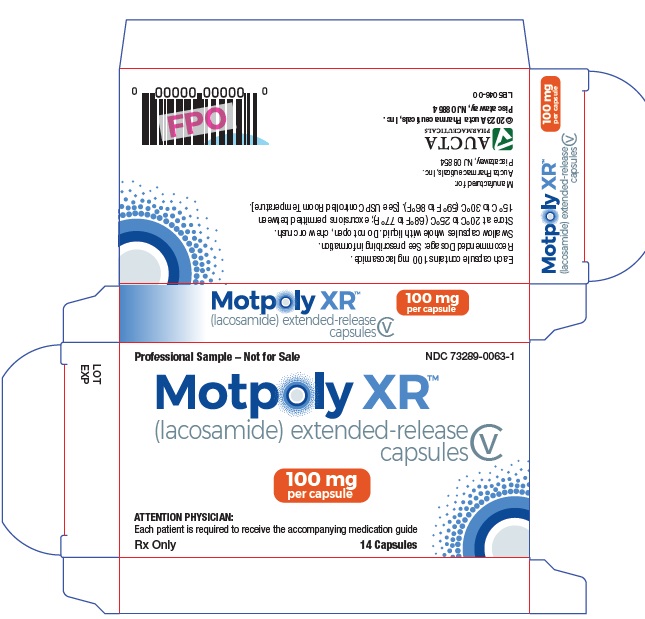
9PRINCIPAL DISPLAY PANEL - Physician Samples
Professional Sample – Not for Sale
NDC 73289-0063-1
Motpoly XR (lacosamide) extended-release capsules
CV
100 mg per capsule
ATTENTION PHYSICIAN:
Each patient is required to receive
the accompanying Medication Guide.
Each patient is required to receive
the accompanying Medication Guide.
Rx Only 14 Capsules
Each capsule contains 100 mg lacosamide.
Manufactured for:
Aucta Pharmaceuticals, Inc.
Piscataway, NJ 08854