Brand Name
Procardia
Generic Name
NIFEdipine
View Brand Information FDA approval date: September 06, 1989
Classification: Dihydropyridine Calcium Channel Blocker
Form: Tablet, Capsule
What is Procardia (NIFEdipine)?
I. Vasospastic Angina Nifedipine Extended-Release Tablets, USP are indicated for the management of vasospastic angina confirmed by any of the following criteria: 1) classical pattern of angina at rest accompanied by ST segment elevation, 2) angina or coronary artery spasm provoked by ergonovine, or 3) angiographically demonstrated coronary artery spasm. In those patients who have had angiography, the presence of significant fixed obstructive disease is not incompatible with the diagnosis of vasospastic angina, provided that the above criteria are satisfied. Nifedipine Extended-Release Tablets, USP may also be used where the clinical presentation suggests a possible vasospastic component, but where vasospasm has not been confirmed, e.g., where pain has a variable threshold on exertion, or in unstable angina where electrocardiographic findings are compatible with intermittent vasospasm, or when angina is refractory to nitrates and/or adequate doses of beta blockers. II. Chronic Stable Angina Nifedipine Extended-Release Tablets, USP are indicated for the management of chronic stable angina without evidence of vasospasm in patients who remain symptomatic despite adequate doses of beta blockers and/or organic nitrates or who cannot tolerate those agents. In chronic stable angina , nifedipine has been effective in controlled trials of up to eight weeks duration in reducing angina frequency and increasing exercise tolerance, but confirmation of sustained effectiveness and evaluation of long-term safety in these patients is incomplete. Controlled studies in small numbers of patients suggest concomitant use of nifedipine and beta-blocking agents may be beneficial in patients with chronic stable angina, but available information is not sufficient to predict with confidence the effects of concurrent treatment, especially in patients with compromised left ventricular function or cardiac conduction abnormalities. When introducing such concomitant therapy, care must be taken to monitor blood pressure closely, since severe hypotension can occur from the combined effects of the drugs. III. Hypertension Nifedipine Extended-Release Tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including Nifedipine Extended-Release Tablets, USP. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension , and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects in black patients, and many antihypertensive drugs have additional approved indications and effects . These considerations may guide selection of therapy. Nifedipine Extended-Release Tablets, USP may be used alone or in combination with other antihypertensive agents.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Procardia (nifedipine)
1DESCRIPTION
Nifedipine is a drug belonging to a class of pharmacological agents known as the calcium channel blockers. Nifedipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, dimethyl ester, C
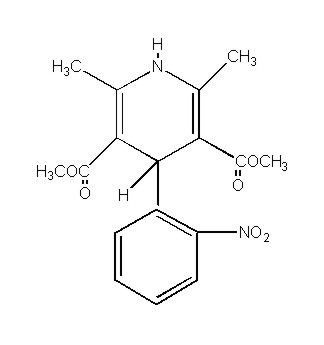
Nifedipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 346.3. PROCARDIA XL is a registered trademark for Nifedipine GITS. Nifedipine GITS (Gastrointestinal Therapeutic System) Tablet is formulated as a once-a-day controlled-release tablet for oral administration to provide 30, 60, or 90 mg of nifedipine.
Each tablet contains 33 mg nifedipine to provide a 30 mg dose.
Inert ingredients in the formulations are: cellulose acetate; hydroxypropyl cellulose; hypromellose; magnesium stearate; polyethylene glycol; polyethylene oxide; red ferric oxide; sodium chloride; titanium dioxide.
1.1System Components and Performance
PROCARDIA XL
PROCARDIA XL Extended Release Tablet is designed to provide nifedipine at an approximately constant rate over 24 hours. This controlled rate of drug delivery into the gastrointestinal lumen is independent of pH or gastrointestinal motility. PROCARDIA XL depends for its action on the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the gastrointestinal tract. Drug delivery is essentially constant as long as the osmotic gradient remains constant, and then gradually falls to zero. Upon swallowing, the biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
2CLINICAL PHARMACOLOGY
Nifedipine is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Nifedipine selectively inhibits calcium ion influx across the cell membrane of cardiac muscle and vascular smooth muscle without altering serum calcium concentrations.
2.1Pharmacokinetics and Metabolism
Nifedipine is completely absorbed after oral administration. Plasma drug concentrations rise at a gradual, controlled rate after a PROCARDIA XL Extended Release Tablet dose and reach a plateau at approximately six hours after the first dose. For subsequent doses, relatively constant plasma concentrations at this plateau are maintained with minimal fluctuations over the 24-hour dosing interval. About a four-fold higher fluctuation index (ratio of peak to trough plasma concentration) was observed with the conventional immediate-release PROCARDIA
Nifedipine is extensively metabolized to highly water-soluble, inactive metabolites, accounting for 60 to 80% of the dose excreted in the urine. The elimination half-life of nifedipine is approximately two hours. Only traces (less than 0.1% of the dose) of unchanged form can be detected in the urine. The remainder is excreted in the feces in metabolized form, most likely as a result of biliary excretion. Thus, the pharmacokinetics of nifedipine are not significantly influenced by the degree of renal impairment. Patients in hemodialysis or chronic ambulatory peritoneal dialysis have not reported significantly altered pharmacokinetics of nifedipine. Since hepatic biotransformation is the predominant route for the disposition of nifedipine, the pharmacokinetics may be altered in patients with chronic liver disease. Patients with hepatic impairment (liver cirrhosis) have a longer disposition half-life and higher bioavailability of nifedipine than healthy volunteers. The degree of serum protein binding of nifedipine is high (92–98%). Protein binding may be greatly reduced in patients with renal or hepatic impairment.
Following intravenous administration, clearance of nifedipine was decreased by 33% in elderly healthy subjects relative to young healthy subjects.
2.2Hemodynamics
Like other slow-channel blockers, nifedipine exerts a negative inotropic effect on isolated myocardial tissue. This is rarely, if ever, seen in intact animals or man, probably because of reflex responses to its vasodilating effects. In man, nifedipine decreases peripheral vascular resistance which leads to a fall in systolic and diastolic pressures, usually minimal in normotensive volunteers (less than 5–10 mm Hg systolic), but sometimes larger. With PROCARDIA XL Extended Release Tablets, these decreases in blood pressure are not accompanied by any significant change in heart rate. Hemodynamic studies in patients with normal ventricular function have generally found a small increase in cardiac index without major effects on ejection fraction, left ventricular end diastolic pressure (LVEDP), or volume (LVEDV). In patients with impaired ventricular function, most acute studies have shown some increase in ejection fraction and reduction in left ventricular filling pressure.
2.3Electrophysiologic Effects
Although, like other members of its class, nifedipine causes a slight depression of sinoatrial node function and atrioventricular conduction in isolated myocardial preparations, such effects have not been seen in studies in intact animals or in man. In formal electrophysiologic studies, predominantly in patients with normal conduction systems, nifedipine has had no tendency to prolong atrioventricular conduction or sinus node recovery time, or to slow sinus rate.
3CONTRAINDICATIONS
Known hypersensitivity reaction to nifedipine.
4ADVERSE EXPERIENCES
Over 1000 patients from both controlled and open trials with PROCARDIA XL Extended Release Tablets in hypertension and angina were included in the evaluation of adverse experiences. All side effects reported during PROCARDIA XL Extended Release Tablet therapy were tabulated independent of their causal relation to medication. The most common side effect reported with PROCARDIA XL was edema which was dose related and ranged in frequency from approximately 10% to about 30% at the highest dose studied (180 mg). Other common adverse experiences reported in placebo-controlled trials include:
Of these, only edema and headache were more common in PROCARDIA XL patients than placebo patients.
The following adverse reactions occurred with an incidence of less than 3.0%. With the exception of leg cramps, the incidence of these side effects was similar to that of placebo alone.
Body as a Whole/Systemic: asthenia, flushing, pain
Cardiovascular: palpitations
Central Nervous System: insomnia, nervousness, paresthesia, somnolence
Dermatologic: pruritus, rash
Gastrointestinal: abdominal pain, diarrhea, dry mouth, dyspepsia, flatulence
Musculoskeletal: arthralgia, leg cramps
Respiratory: chest pain (nonspecific), dyspnea
Urogenital: impotence, polyuria
Cardiovascular: palpitations
Central Nervous System: insomnia, nervousness, paresthesia, somnolence
Dermatologic: pruritus, rash
Gastrointestinal: abdominal pain, diarrhea, dry mouth, dyspepsia, flatulence
Musculoskeletal: arthralgia, leg cramps
Respiratory: chest pain (nonspecific), dyspnea
Urogenital: impotence, polyuria
Other adverse reactions were reported sporadically with an incidence of 1.0% or less. These include:
Body as a Whole/Systemic: face edema, fever, hot flashes, malaise, periorbital edema, rigors
Cardiovascular: arrhythmia, hypotension, increased angina, tachycardia, syncope
Central Nervous System: anxiety, ataxia, decreased libido, depression, hypertonia, hypoesthesia, migraine, paroniria, tremor, vertigo
Dermatologic: alopecia, increased sweating, urticaria, purpura
Gastrointestinal: eructation, gastroesophageal reflux, gum hyperplasia, melena, vomiting, weight increase
Musculoskeletal: back pain, gout, myalgias
Respiratory: coughing, epistaxis, upper respiratory tract infection, respiratory disorder, sinusitis
Special Senses: abnormal lacrimation, abnormal vision, taste perversion, tinnitus
Urogenital/Reproductive: breast pain, dysuria, hematuria, nocturia
Cardiovascular: arrhythmia, hypotension, increased angina, tachycardia, syncope
Central Nervous System: anxiety, ataxia, decreased libido, depression, hypertonia, hypoesthesia, migraine, paroniria, tremor, vertigo
Dermatologic: alopecia, increased sweating, urticaria, purpura
Gastrointestinal: eructation, gastroesophageal reflux, gum hyperplasia, melena, vomiting, weight increase
Musculoskeletal: back pain, gout, myalgias
Respiratory: coughing, epistaxis, upper respiratory tract infection, respiratory disorder, sinusitis
Special Senses: abnormal lacrimation, abnormal vision, taste perversion, tinnitus
Urogenital/Reproductive: breast pain, dysuria, hematuria, nocturia
Adverse experiences which occurred in less than 1 in 1000 patients cannot be distinguished from concurrent disease states or medications.
The following adverse experiences, reported in less than 1% of patients, occurred under conditions (e.g., open trials, marketing experience) where a causal relationship is uncertain: gastrointestinal irritation, gastrointestinal bleeding, gynecomastia.
Gastrointestinal obstruction resulting in hospitalization and surgery, including the need for bezoar removal, has occurred in association with PROCARDIA XL, even in patients with no prior history of gastrointestinal disease (see
Cases of tablet adherence to the gastrointestinal wall with ulceration have been reported, some requiring hospitalization and intervention.
In multiple-dose U.S. and foreign controlled studies with nifedipine capsules in which adverse reactions were reported spontaneously, adverse effects were frequent but generally not serious and rarely required discontinuation of therapy or dosage adjustment. Most were expected consequences of the vasodilator effects of nifedipine.
There is also a large uncontrolled experience in over 2100 patients in the United States. Most of the patients had vasospastic or resistant angina pectoris, and about half had concomitant treatment with beta-adrenergic blocking agents. The relatively common adverse events were similar in nature to those seen with PROCARDIA XL.
In addition, more serious adverse events were observed, not readily distinguishable from the natural history of the disease in these patients. It remains possible, however, that some or many of these events were drug related. Myocardial infarction occurred in about 4% of patients and congestive heart failure or pulmonary edema in about 2%. Ventricular arrhythmias or conduction disturbances each occurred in fewer than 0.5% of patients.
In a subgroup of over 1000 patients receiving PROCARDIA with concomitant beta blocker therapy, the pattern and incidence of adverse experiences was not different from that of the entire group of PROCARDIA-treated patients (see
In a subgroup of approximately 250 patients with a diagnosis of congestive heart failure as well as angina, dizziness or lightheadedness, peripheral edema, headache, or flushing each occurred in one in eight patients. Hypotension occurred in about one in 20 patients. Syncope occurred in approximately one patient in 250. Myocardial infarction or symptoms of congestive heart failure each occurred in about one patient in 15. Atrial or ventricular dysrhythmias each occurred in about one patient in 150.
In post-marketing experience, there have been rare reports of exfoliative dermatitis caused by nifedipine. There have been rare reports of exfoliative or bullous skin adverse events (such as erythema multiforme, Stevens-Johnson Syndrome, and toxic epidermal necrolysis) and photosensitivity reactions. Acute generalized exanthematous pustulosis also has been reported.
5OVERDOSAGE
Experience with nifedipine overdosage is limited. Generally, overdosage with nifedipine leading to pronounced hypotension calls for active cardiovascular support, including monitoring of cardiovascular and respiratory function, elevation of extremities, judicious use of calcium infusion, pressor agents, and fluids. Clearance of nifedipine would be expected to be prolonged in patients with impaired liver function. Since nifedipine is highly protein-bound, dialysis is not likely to be of any benefit.
There has been one reported case of massive overdosage with PROCARDIA XL Extended Release Tablets. The main effects of ingestion of approximately 4800 mg of PROCARDIA XL in a young man attempting suicide as a result of cocaine-induced depression was initial dizziness, palpitations, flushing, and nervousness. Within several hours of ingestion, nausea, vomiting, and generalized edema developed. No significant hypotension was apparent at presentation, 18 hours post-ingestion. Electrolyte abnormalities consisted of a mild, transient elevation of serum creatinine, and modest elevations of LDH and CPK, but normal SGOT. Vital signs remained stable, no electrocardiographic abnormalities were noted, and renal function returned to normal within 24 to 48 hours with routine supportive measures alone. No prolonged sequelae were observed.
The effect of a single 900 mg ingestion of PROCARDIA capsules in a depressed anginal patient also on tricyclic antidepressants was loss of consciousness within 30 minutes of ingestion, and profound hypotension, which responded to calcium infusion, pressor agents, and fluid replacement. A variety of ECG abnormalities were seen in this patient with a history of bundle branch block, including sinus bradycardia and varying degrees of AV block. These dictated the prophylactic placement of a temporary ventricular pacemaker, but otherwise resolved spontaneously. Significant hyperglycemia was seen initially in this patient, but plasma glucose levels rapidly normalized without further treatment.
A young hypertensive patient with advanced renal failure ingested 280 mg of PROCARDIA capsules at one time, with resulting marked hypotension responding to calcium infusion and fluids. No AV conduction abnormalities, arrhythmias, or pronounced changes in heart rate were noted, nor was there any further deterioration in renal function.
6DOSAGE AND ADMINISTRATION
Dosage must be adjusted according to each patient's needs. Therapy for either hypertension or angina should be initiated with 30 or 60 mg once daily. PROCARDIA XL Extended Release Tablets should be swallowed whole and should not be bitten or divided. In general, titration should proceed over a 7–14 day period so that the physician can fully assess the response to each dose level and monitor blood pressure before proceeding to higher doses. Since steady-state plasma levels are achieved on the second day of dosing, titration may proceed more rapidly, if symptoms so warrant, provided the patient is assessed frequently. Titration to doses above 120 mg are not recommended.
Angina patients controlled on PROCARDIA capsules alone or in combination with other antianginal medications may be safely switched to PROCARDIA XL Extended Release Tablets at the nearest equivalent total daily dose (e.g., 30 mg t.i.d. of PROCARDIA capsules may be changed to 90 mg once daily of PROCARDIA XL Extended Release Tablets). Subsequent titration to higher or lower doses may be necessary and should be initiated as clinically warranted. Experience with doses greater than 90 mg in patients with angina is limited. Therefore, doses greater than 90 mg should be used with caution and only when clinically warranted.
Avoid co-administration of nifedipine with grapefruit juice (see
No "rebound effect" has been observed upon discontinuation of PROCARDIA XL Extended Release Tablets. However, if discontinuation of nifedipine is necessary, sound clinical practice suggests that the dosage should be decreased gradually with close physician supervision.
Care should be taken when dispensing PROCARDIA XL to assure that the extended release dosage form has been prescribed.
6.1Co-Administration with Other Antianginal Drugs
Sublingual nitroglycerin may be taken as required for the control of acute manifestations of angina, particularly during nifedipine titration. See
7HOW SUPPLIED
PROCARDIA XL Extended Release Tablets are supplied to provide 30 mg, 60 mg and 90 mg round, biconvex, rose-pink, film-coated tablets in:
8PRINCIPAL DISPLAY PANEL - 30 mg Tablet Bottle Label
Pfizer
NDC 0069-2650-66
NDC 0069-2650-66
Procardia XL®
(nifedipine)
(nifedipine)
30 mg GITS*
Extended Release Tablets
100 Tablets

9PRINCIPAL DISPLAY PANEL - 60 mg Tablet Bottle Label
Pfizer
NDC 0069-2660-66
NDC 0069-2660-66
Procardia XL®
(nifedipine)
(nifedipine)
60 mg GITS*
Extended Release Tablets
100 Tablets

10PRINCIPAL DISPLAY PANEL - 90 mg Tablet Bottle Label
Pfizer
NDC 0069-2670-66
NDC 0069-2670-66
Procardia XL®
(nifedipine)
(nifedipine)
90 mg GITS*
Extended Release Tablets
100 Tablets
