Generic Name
Ketorolac Tromethamine
Brand Names
Acuvail, Sprix, Toronova SUIK, Acular, Ketorocaine, Strenza, Toronova
FDA approval date: December 01, 1992
Classification: Nonsteroidal Anti-inflammatory Drug
Form: Injection, Spray, Tablet, Kit, Solution
What is Acuvail (Ketorolac Tromethamine)?
Ketorolac tromethamine ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery. Ketorolac tromethamine ophthalmic solution is a nonsteroidal, anti-inflammatory drug indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ACUVAIL (ketorolac tromethamine)
1INDICATIONS AND USAGE
ACUVAIL® is indicated for the treatment of pain and inflammation following cataract surgery.
2DOSAGE FORMS AND STRENGTHS
ACUVAIL (ketorolac tromethamine ophthalmic solution) is a sterile, clear, and colorless to pale yellow ophthalmic solution containing 0.45% (4.5 mg/mL) ketorolac tromethamine in a single-dose vial.
3CONTRAINDICATIONS
ACUVAIL is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Delayed Healing
- Cross-Sensitivity or Hypersensitivity
- Increased Bleeding Time
- Corneal Effects
4.1Clinical TrialsExperience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions were reported in 1% to 6% of patients and included increased intraocular pressure, conjunctival hyperemia and/or hemorrhage, corneal edema, ocular pain, headache, tearing and vision blurred. Some of these reactions may be the consequence of the cataract surgical procedure.
Other adverse reactions occurring during treatment with ketorolac tromethamine ophthalmic solutions included allergic reactions (including eye swelling, hyperemia, and pruritis).
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ketorolac tromethamine ophthalmic solutions in clinical practice. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug.
Eye Disorders: Corneal erosion, corneal perforation, corneal thinning and corneal melt, epithelial breakdown [see Warnings and Precautions (5.2, 5.4)], and ulcerative keratitis.
Respiratory, Thoracic and Mediastinal Disorders: Bronchospasm, exacerbation of asthma.
5DESCRIPTION
ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for topical ophthalmic use. Its chemical name is (±)-5-Benzoyl-2,3-dihydro-1
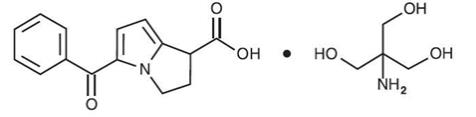
ACUVAIL
Each mL of ACUVAIL ophthalmic solution contains:
6CLINICAL STUDIES
Two multicenter, randomized, double-masked, parallel group comparison studies including approximately 500 patients were conducted to evaluate the effects of ACUVAIL on anterior chamber cell and flare, and ocular pain relief following cataract extraction with posterior chamber intraocular lens (IOL) implantation. Results of these studies indicated that patients receiving ACUVAIL had a significantly higher incidence of clearing of anterior chamber inflammation 53% (167/318) versus patients receiving vehicle 26% (41/155) at day 14.
ACUVAIL was also significantly superior to vehicle in resolving ocular pain. On Day 1 post cataract surgery, 72% (233/322) of patients in the ACUVAIL group were pain free compared to 40% (62/156) of patients in the vehicle group.
Results from clinical studies indicate that ketorolac tromethamine has no significant effect upon intraocular pressure; however, changes in intraocular pressure may occur following cataract surgery.
7HOW SUPPLIED/STORAGE AND HANDLING
ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is supplied as a sterile, clear, and colorless to pale yellow ophthalmic solution in clear, low density polyethylene (LDPE), single-dose vials packaged in 3 foil pouches, 10 vials per pouch:
30 Single-Dose Vials 0.4 mL each (Carton containing 3 pouches): NDC 0023-3507-31
Storage: Store at 15oC to 30ºC (59oF to 86ºF). Store the vials in the pouch, protected from light. Fold pouch ends closed. The solution from one individual single-dose vial is to be used immediately after opening. The remaining vial contents should be discarded.
8PATIENT COUNSELING INFORMATION
Slow or Delayed Healing
Advise patients of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs).
Avoiding Contamination of the Product
Instruct patients that the solution from one individual single-dose vial is to be used immediately after opening for administration to the affected eye. The remaining vial contents should be discarded.
Instruct patients to avoid allowing the tip of the single-dose vial to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections or cause injury to the eye. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Store the vials in the pouch, protected from light. Fold pouch ends closed.
Contact Lens Wear
Advise patients that ACUVAIL should not be administered while wearing contact lenses.
Intercurrent Ocular Conditions
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, they should immediately seek their physician’s advice concerning the continued use of ACUVAIL.
Concomitant Topical Ocular Therapy
Advise patients that if more than one topical ophthalmic medication is being used, the medicines should be administered at least 5 minutes apart.
Distributed by:
© 2024 AbbVie. All rights reserved.

V3.0USPI3507
9PRINCIPAL DISPLAY PANEL
NDC 0023-3507-31
abbvie
ACUVAIL®
(ketorolac tromethamine
ophthalmic solution)0.45%
FOT TOPICAL OPHTHALMIC USE
Contains No Anti-Microbial Preservatives
FOR SINGLE-DOSE ONLY
Rx Only
sterile
30 Single-Dose Vials. Discard After Use. 0.4mL Each
ACUVAIL®
(ketorolac tromethamine
ophthalmic solution)0.45%
FOT TOPICAL OPHTHALMIC USE
Contains No Anti-Microbial Preservatives
FOR SINGLE-DOSE ONLY
Rx Only
sterile
30 Single-Dose Vials. Discard After Use. 0.4mL Each
10PRINCIPAL DISPLAY PANEL
NDC 0023-3507-30
