Brand Name
Captopril
View Brand InformationFDA approval date: February 13, 1996
Classification: Angiotensin Converting Enzyme Inhibitor
Form: Tablet
What is Captopril?
Hypertension: Captopril tablets, USP, are indicated for the treatment of hypertension. In using captopril tablets, USP, consideration should be given to the risk of neutropenia/agranulocytosis. Captopril tablets, USP may be used as initial therapy for patients with normal renal function, in whom the risk is relatively low. In patients with impaired renal function, particularly those with collagen vascular disease, captopril should be reserved for hypertensives who have either developed unacceptable side effects on other drugs, or have failed to respond satisfactorily to drug combinations. Captopril tablets, USP are effective alone and in combination with other antihypertensive agents, especially thiazidetype diuretics. The blood pressure lowering effects of captopril and thiazides are approximately additive. Heart Failure: Captopril tablets, USP are indicated in the treatment of congestive heart failure usually in combination with diuretics and digitalis. The beneficial effect of captopril in heart failure does not require the presence of digitalis, however, most controlled clinical trial experience with captopril has been in patients receiving digitalis, as well as diuretic treatment. Left Ventricular Dysfunction After Myocardial Infarction: Captopril tablets, USP are indicated to improve survival following myocardial infarction in clinically stable patients with left ventricular dysfunction manifested as an ejection fraction ≤ 40% and to reduce the incidence of overt heart failure and subsequent hospitalizations for congestive heart failure in these patients. Diabetic Nephropathy: Captopril tablets, USP are indicated for the treatment of diabetic nephropathy in patients with type I insulin-dependent diabetes mellitus and retinopathy. Captopril tablets, USP decreases the rate of progression of renal insufficiency and development of serious adverse clinical outcomes . In considering use of captopril tablets, USP, it should be noted that in controlled trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, ACE inhibitors cause a higher rate of angioedema in black than in non-black patients.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Captopril (Captopril)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue captopril tablets as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See
1DESCRIPTION
Captopril Tablets, USP are a specific competitive inhibitor of angiotensin I-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I to angiotensin II.
Captopril is designated chemically as 1-[(2S)-3-mercapto-2-methylpropionyl]-L-proline and has the following structural formula:
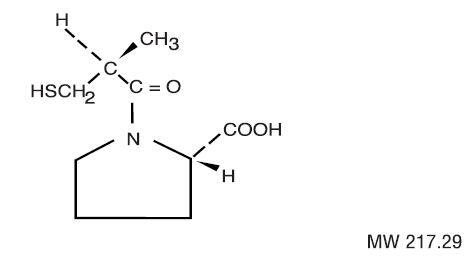
Captopril is a white to off-white crystalline powder that may have a slight sulfurous odor; it is soluble in water (approx. 160 mg/mL), methanol, and ethanol and sparingly soluble in chloroform and ethyl acetate.
Each tablet for oral administration contains 12.5 mg, 25 mg, 50 mg or 100 mg of captopril. In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, microcrystalline cellulose, sodium starch glycolate (derived from potato), starch (derived from corn), and stearic acid.
2CONTRAINDICATIONS
Captopril tablets are contraindicated in patients who are hypersensitive to this product or any other angiotensin-converting enzyme inhibitor (e.g., a patient who has experienced angioedema during therapy with any other ACE inhibitor).
Do not co-administer aliskiren with captopril tablets in patients with diabetes (see
Captopril tablets are contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer captopril tablets within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor (see
3ADVERSE REACTIONS
Reported incidences are based on clinical trials involving approximately 7,000 patients.
Each of the following has been reported in approximately 1 to 2 of 1,000 patients and are of uncertain relationship to drug use: renal insufficiency, renal failure, nephrotic syndrome, polyuria, oliguria, and urinary frequency.
Hematologic: Neutropenia/agranulocytosis has occurred (see WARNINGS). Cases of anemia, thrombocytopenia, and pancytopenia have been reported.
Dermatologic: Rash, often with pruritus, and sometimes with fever, arthralgia, and eosinophilia, occurred in about 4 to 7 (depending on renal status and dose) of 100 patients, usually during the first four weeks of therapy. It is usually maculopapular, and rarely urticarial. The rash is usually mild and disappears within a few days of dosage reduction, short-term treatment with an antihistaminic agent, and/or discontinuing therapy; remission may occur even if captopril is continued. Pruritus, without rash, occurs in about 2 of 100 patients. Between 7 and 10 percent of patients with skin rash have shown an eosinophilia and/or positive ANA titers. A reversible associated pemphigoid-like lesion, and photosensitivity, have also been reported.
Flushing or pallor has been reported in 2 to 5 of 1,000 patients.
Cardiovascular: Hypotension may occur; see WARNINGS and PRECAUTIONS [Drug Interactions]for discussion of hypotension with captopril therapy.
Tachycardia, chest pain, and palpitations have each been observed in approximately 1 of 100 patients.
Angina pectoris, myocardial infarction, Raynaud’s syndrome, and congestive heart failure have each occurred in 2 to 3 of 1,000 patients.
Dysgeusia: Approximately 2 to 4 (depending on renal status and dose) of 100 patients developed a diminution or loss of taste perception. Taste impairment is reversible and usually self-limited (2 to 3 months) even with continued drug administration. Weight loss may be associated with the loss of taste.
Angioedema: Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx has been reported in approximately one in 1,000 patients. Angioedema involving the upper airways has caused fatal airway obstruction (see WARNINGS: Head and Neck Angioedema, Intestinal Angioedemaand PRECAUTIONS: Information for Patients).
Cough: Cough has been reported in 0.5 to 2% of patients treated with captopril in clinical trials (See PRECAUTIONS: General, Cough).
The following have been reported in about 0.5 to 2 percent of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials: gastric irritation, abdominal pain, nausea, vomiting, diarrhea, anorexia, constipation, aphthous ulcers, peptic ulcer, dizziness, headache, malaise, fatigue, insomnia, dry mouth, dyspnea, alopecia, paresthesias.
Other clinical adverse effects reported since the drug was marketed are listed below by body system. In this setting, an incidence or causal relationship cannot be accurately determined.
Body as a Whole: Anaphylactoid reactions (See WARNINGS: Anaphylactoid and Possible Related Reactionsand PRECAUTIONS: Hemodialysis).
General: Asthenia, gynecomastia.
Cardiovascular: Cardiac arrest, cerebrovascular accident/insufficiency, rhythm disturbances, orthostatic hypotension, syncope.
Dermatologic: Bullous pemphigus, erythema multiforme (including Stevens-Johnson syndrome), exfoliative dermatitis.
Gastrointestinal: Pancreatitis, glossitis, dyspepsia.
Hematologic: Anemia, including aplastic and hemolytic.
Hepatobiliary: Jaundice, hepatitis, including rare cases of necrosis, cholestasis.
Metabolic: Symptomatic hyponatremia.
Musculoskeletal: Myalgia, myasthenia.
Nervous/Psychiatric: Ataxia, confusion, depression, nervousness, somnolence.
Respiratory: Bronchospasm, eosinophilic pneumonitis, rhinitis.
Special Senses: Blurred vision.
Urogenital: Impotence.
As with other ACE inhibitors, a syndrome has been reported which may include: fever, myalgia, arthralgia, interstitial nephritis, vasculitis, rash or other dermatologic manifestations, eosinophilia and an elevated ESR.
3.1Altered Laboratory Findings
Serum Electrolytes: Hyperkalemia: Small increases in serum potassium, especially in patients with renal impairment (see PRECAUTIONS).
Hyponatremia: Particularly in patients receiving a low sodium diet or concomitant diuretics.
BUN/Serum Creatinine: Transient elevations of BUN or serum creatinine especially in volume or salt depleted patients or those with renovascular hypertension may occur. Rapid reduction of longstanding or markedly elevated blood pressure can result in decreases in the glomerular filtration rate and, in turn, lead to increases in BUN or serum creatinine.
Hematologic: A positive ANA has been reported.
Liver Function Tests: Elevations of liver transaminases, alkaline phosphatase, and serum bilirubin have occurred.
To report SUSPECTED ADVERSE REACTIONS, contact West-Ward Pharmaceuticals Corp. at 1-877-233-2001, or FDA at 1-800-FDA-1088 or
4OVERDOSAGE
Correction of hypotension would be of primary concern. Volume expansion with an intravenous infusion of normal saline is the treatment of choice for restoration of blood pressure.
While captopril may be removed from the adult circulation by hemodialysis, there is inadequate data concerning the effectiveness of hemodialysis for removing it from the circulation of neonates or children. Peritoneal dialysis is not effective for removing captopril; there is no information concerning exchange transfusion for removing captopril from the general circulation.
5DOSAGE AND ADMINISTRATION
Captopril tablets should be taken one hour before meals. Dosage must be individualized.
Hypertension: Initiation of therapy requires consideration of recent antihypertensive drug treatment, the extent of blood pressure elevation, salt restriction, and other clinical circumstances. If possible, discontinue the patient’s previous antihypertensive drug regimen for one week before starting captopril tablets.
The initial dose of captopril tablets is 25 mg b.i.d. or t.i.d. If satisfactory reduction of blood pressure has not been achieved after one or two weeks, the dose may be increased to 50 mg b.i.d. or t.i.d. Concomitant sodium restriction may be beneficial when captopril tablets are used alone.
The dose of captopril tablets in hypertension usually does not exceed 50 mg t.i.d. Therefore, if the blood pressure has not been satisfactorily controlled after one to two weeks at this dose, (and the patient is not already receiving a diuretic), a modest dose of a thiazide-type diuretic (e.g., hydrochlorothiazide, 25 mg daily), should be added. The diuretic dose may be increased at one to two-week intervals until its highest usual antihypertensive dose is reached.
If captopril tablets are being started in a patient already receiving a diuretic, captopril tablets therapy should be initiated under close medical supervision (see
If further blood pressure reduction is required, the dose of captopril tablets may be increased to 100 mg b.i.d. or t.i.d. and then, if necessary, to 150 mg b.i.d. or t.i.d. (while continuing the diuretic). The usual dose range is 25 to 150 mg b.i.d. or t.i.d. A maximum daily dose of 450 mg captopril tablets should not be exceeded.
For patients with severe hypertension (e.g., accelerated or malignant hypertension), when temporary discontinuation of current antihypertensive therapy is not practical or desirable, or when prompt titration to more normotensive blood pressure levels is indicated, diuretic should be continued but other current antihypertensive medication stopped and captopril tablets dosage promptly initiated at 25 mg b.i.d. or t.i.d., under close medical supervision.
When necessitated by the patient’s clinical condition, the daily dose of captopril tablets may be increased every 24 hours or less under continuous medical supervision until a satisfactory blood pressure response is obtained or the maximum dose of captopril tablets is reached. In this regimen, addition of a more potent diuretic, e.g., furosemide, may also be indicated.
Beta-blockers may also be used in conjunction with captopril tablets therapy (see
Heart Failure: Initiation of therapy requires consideration of recent diuretic therapy and the possibility of severe salt/volume depletion. In patients with either normal or low blood pressure, who have been vigorously treated with diuretics and who may be hyponatremic and/or hypovolemic, a starting dose of 6.25 or 12.5 mg t.i.d. may minimize the magnitude or duration of the hypotensive effect (see WARNINGS: Hypotension); for these patients, titration to the usual daily dosage can then occur within the next several days.
For most patients the usual initial daily dosage is 25 mg t.i.d. After a dose of 50 mg t.i.d. is reached, further increases in dosage should be delayed, where possible, for at least two weeks to determine if a satisfactory response occurs. Most patients studied have had a satisfactory clinical improvement at 50 or 100 mg t.i.d. A maximum daily dose of 450 mg of captopril tablets should not be exceeded.
Captopril tablets should generally be used in conjunction with a diuretic and digitalis. Captopril tablets therapy must be initiated under very close medical supervision.
Left Ventricular Dysfunction After Myocardial Infarction: The recommended dose for long-term use in patients following a myocardial infarction is a target maintenance dose of 50 mg t.i.d.
Therapy may be initiated as early as three days following a myocardial infarction. After a single dose of 6.25 mg, captopril tablets therapy should be initiated at 12.5 mg t.i.d. Captopril tablets should then be increased to 25 mg t.i.d. during the next several days and to a target dose of 50 mg t.i.d. over the next several weeks as tolerated (see
Captopril tablets may be used in patients treated with other post-myocardial infarction therapies, e.g., thrombolytics, aspirin, beta blockers.
Diabetic Nephropathy: The recommended dose of captopril tablets for long term use to treat diabetic nephropathy is 25 mg t.i.d.
Other antihypertensives such as diuretics, beta blockers, centrally acting agents or vasodilators may be used in conjunction with captopril tablets if additional therapy is required to further lower blood pressure.
Dosage Adjustment in Renal Impairment: Because captopril tablets are excreted primarily by the kidneys, excretion rates are reduced in patients with impaired renal function. These patients will take longer to reach steady-state captopril levels and will reach higher steady-state levels for a given daily dose than patients with normal renal function. Therefore, these patients may respond to smaller or less frequent doses.
Accordingly, for patients with significant renal impairment, initial daily dosage of captopril tablets should be reduced, and smaller increments utilized for titration, which should be quite slow (one- to two-week intervals). After the desired therapeutic effect has been achieved, the dose should be slowly back-titrated to determine the minimal effective dose. When concomitant diuretic therapy is required, a loop diuretic (e.g., furosemide), rather than a thiazide diuretic, is preferred in patients with severe renal impairment (see
6HOW SUPPLIED
Captopril Tablets, USP are available as follows:
12.5 mg - White, Round Tablets; Debossed "W-7" on one side and Scored on the other side.
25 mg - White, Round Tablets; Debossed "WW 172" on one side and Quadrisect Scored on the other side.
All Captopril Tablets, USP are white and may exhibit a slight sulfurous odor.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
7PACKAGING INFORMATION
American Health Packaging unit dose blisters (see
Distributed by:
8430421/0320F
8Package/Label Display Panel – Carton – 12.5 mg

NDC 60687-
Captopril
Tablets, USP
Tablets, USP
12.5 mg
30 Tablets (3 x 10) Rx Only
Each Tablet Contains:
Captopril, USP.................................................................. 12.5 mg
Captopril, USP.................................................................. 12.5 mg
Usual Dosage: See full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
Distributed by: American Health Packaging, Columbus, Ohio 43217
730421
9Package/Label Display Panel – Blister – 12.5 mg

Captopril
12.5 mg
10Package/Label Display Panel – Carton - 25 mg

NDC 60687-
Captopril
Tablets, USP
Tablets, USP
25 mg
30 Tablets (3 x 10) Rx Only
Each Tablet Contains:
Captopril, USP..................................................................... 25 mg
Captopril, USP..................................................................... 25 mg
Usual Dosage: See full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
Distributed by: American Health Packaging, Columbus, Ohio 43217
731521
11Package/Label Display Panel – Blister - 25 mg

Captopril
25 mg