Generic Name
Nitisinone
Brand Names
Orfadin, NITYR, Harliku
FDA approval date: June 01, 2016
Classification: 4-Hydroxyphenyl-Pyruvate Dioxygenase Inhibitor
Form: Tablet, Suspension, Capsule
What is Orfadin (Nitisinone)?
Nitisinone capsules are indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine. Nitisinone capsules are a hydroxy-phenylpyruvate dioxygenase inhibitor indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ORFADIN (nitisinone)
1INDICATIONS AND USAGE
ORFADIN
2DOSAGE FORMS AND STRENGTHS
- Capsules: 2 mg, 5 mg, 10 mg and 20 mg white capsules imprinted with “NTBC” followed by “2 mg”, “5 mg”, “10 mg” or ”20 mg”, indicating the actual amount of nitisinone in each capsule.
- Oral suspension: 4 mg/mL, a white, slightly viscous opaque suspension.
3CONTRAINDICATIONS
None.
4DRUG INTERACTIONS
Nitisinone is a moderate CYP2C9 inhibitor, a weak CYP2E1 inducer and an inhibitor of OAT1/OAT3.
5OVERDOSAGE
Accidental ingestion of ORFADIN by individuals eating normal diets not restricted in tyrosine and phenylalanine will result in elevated tyrosine levels. In healthy subjects given a single 1 mg/kg dose of nitisinone, the plasma tyrosine level reached a maximum of 1200 micromol/L at 48 to 120 hours after dosing. After a washout period of 14 days, the mean value of plasma tyrosine was still 808 micromol/L. Fasted follow-up samples obtained from volunteers several weeks later showed tyrosine values back to normal. There were no reports of changes in vital signs or laboratory data of any clinical significance. One patient reported sensitivity to sunlight. Hyper-tyrosinemia has been reported with ORFADIN treatment
6DESCRIPTION
ORFADIN contains nitisinone, which is a hydroxyphenyl-pyruvate dioxygenase inhibitor indicated as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1 (HT-1).
Nitisinone occurs as white to yellowish-white, crystalline powder. It is practically insoluble in water, soluble in 2M sodium hydroxide and in methanol, and sparingly soluble in alcohol.
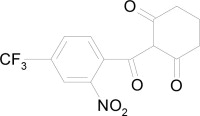
Chemically, nitisinone is 2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione, and the structural formula is:
Capsules: Hard, white-opaque capsule, marked as 2 mg, 5 mg, 10 mg or 20 mg strengths of nitisinone, intended for oral administration. Each capsule contains 2 mg, 5 mg, 10 mg or 20 mg nitisinone, plus pre-gelatinized starch. The capsule shell is gelatin and titanium dioxide and the imprint is an iron oxide.
Oral suspension: 4 mg/mL, a white, slightly viscous opaque suspension. The inactive ingredients are hydroxypropyl methylcellulose, glycerol, polysorbate 80, sodium benzoate, citric acid monohydrate, trisodium citrate dihydrate, strawberry aroma (artificial) and purified water.
Glycerol: Each mL contains 500 mg.
Sodium: Each mL contains 0.7 mg (0.03 mEq).
7CLINICAL STUDIES
The efficacy and safety of ORFADIN in patients with HT-1 was evaluated in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 22 years at enrollment (median age 9 months). Patients were diagnosed with HT-1 by the presence of succinylacetone in the urine or plasma. All patients were treated with ORFADIN at a starting dose of 0.3 to 0.5 mg/kg twice daily, and the dose was increased in some patients to 1 mg/kg twice daily based on weight, liver and kidney function tests, platelet count, serum amino acids, urinary phenolic acid, plasma and urine succinylacetone, erythrocyte PBG-synthase, and urine 5-ALA. The median duration of treatment was 22 months (range less than 1 month to 80 months). Efficacy was assessed by comparison of survival and incidence of liver transplant to historical controls.
For patients presenting with HT-1 younger than 2 months of age who were treated with dietary restriction and nitisinone, 2- and 4-year survival probabilities were 88% and 88%, respectively. Data from historical controls showed that patients presenting with HT-1 at younger than 2 months of age and treated with dietary restriction alone had 2- and 4-year survival probabilities of 29% and 29%, respectively. For patients presenting with HT-1 between 2 months and 6 months of age who were treated with dietary restriction and nitisinone, 2- and 4-year survival probabilities were 94% and 94%, respectively. Data for historical controls showed that patients presenting with HT-1 between 2 months and 6 months of age treated with dietary restriction alone had 2- and 4-year survival probabilities of 74% and 60%, respectively.
The effects of nitisinone on urine and plasma succinylacetone, porphyrin metabolism, and urinary alpha-1-microglobulin were also assessed in this clinical study.
Porphyria-like crisis were reported in 3 patients (0.3% of cases per year) during the clinical study. This compares to an incidence of 5 to 20% of cases per year expected as part of the natural history of the disorder. An assessment of porphyria-like crises was performed because these events are commonly reported in patients with HT-1 who are not treated with nitisinone.
Urinary alpha-1-microglobulin, a proposed marker of proximal tubular dysfunction, was measured in 100 patients at baseline. The overall median pretreatment level was 4.3 grams/mol creatinine. After one year of treatment in a subgroup of patients (N=100), overall median alpha-1-microglobulin decreased by 1.5 grams/mol creatinine. In patients 24 months of age and younger in whom multiple values were available (N=65), median alpha-1-microglobulin levels decreased from 5.0 to 3.0 grams/mol creatinine (reference value for age less than or equal to 12 grams/mol creatinine). In patients older than 24 months in whom multiple values were available (N=35), median alpha-1-microglobulin levels decreased from 2.8 to 2.0 grams/mol creatinine (reference for age less than or equal to 6 grams/mol creatinine).
The long term effect of nitisinone on hepatic function was not assessed.
8HOW SUPPLIED/STORAGE AND HANDLING
Capsules: White capsules marked in black with "NTBC" and identified as 2 mg, 5 mg, 10 mg or 20 mg strengths of nitisinone. The capsules are packed in a high density (HD) polyethylene container with a tamper-resistant low density (LD) polyethylene snap-on cap. Each bottle contains 60 capsules.
- 2 mg white capsules imprinted "NTBC 2 mg" in black ink, NDC 66658-102-60
- 5 mg white capsules imprinted "NTBC 5 mg" in black ink, NDC 66658-105-60
- 10 mg white capsules imprinted "NTBC 10 mg" in black ink, NDC 66658-110-60
- 20 mg white capsules imprinted "NTBC 20 mg" in black ink, NDC 66658-120-60
- Store refrigerated at 2° to 8°C (36° to 46°F). Alternatively, patients/caregivers may store ORFADIN capsules at room temperature up to 25°C (77°F) for up to 45 days. If not used within 45 days, discard ORFADIN capsules.
Oral suspension: White, slightly viscous opaque suspension. 1 mL contains 4 mg of nitisinone. The suspension is provided in a 100 mL brown bottle (type III glass) with a white child resistant HDPE screw cap with sealing and tamper evidence. Each bottle contains 90 mL oral suspension.
- Oral suspension 4 mg/mL, NDC 66658-204-90
- Store refrigerated at 2°C to 8°C (36°F to 46°F) prior to first use. Do not freeze. Store upright.
- After first opening, store the product at room temperature (up to 25°C (77°F)) for up to 60 days. If not used within 60 days, discard unused portion. The discard after date should be noted on the bottle
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (