Generic Name
Gabapentin
Brand Names
Gaba 300-EZS, Gralise, Neurontin, Gabarone
FDA approval date: December 30, 1993
Classification: Anti-epileptic Agent
Form: Tablet, Kit, Suspension, Capsule, Solution
What is Gaba 300-EZS (Gabapentin)?
Gabapentinis indicated for: Management of post herpetic neuralgia in adults, Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy Gabapentin is indicated for: · Post herpetic neuralgia in adults · Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Gaba 300-EZS (Gabapentin)
1INDICATIONS AND USAGE
Gabapentin capsules are indicated for:
- Management of postherpetic neuralgia in adults
- Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy
2DOSAGE FORMS AND STRENGTHS
Capsules:
• 100 mg: white and light brown capsule printed with
• 300 mg: yellow and light brown capsule printed with
• 400 mg: orange and light brown capsule printed with
3CONTRAINDICATIONS
Gabapentin capsules are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients.
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections:
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Anaphylaxis and Angioedema
- Somnolence/Sedation and Dizziness
- Withdrawal Precipitated Seizure, Status Epilepticus
- Suicidal Behavior and Ideation
- Neuropsychiatric Adverse Reactions (Pediatric Patients 3 to 12 Years of Age)
- Sudden and Unexplained Death in Patients with Epilepsy
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Postherpetic Neuralgia
The most common adverse reactions associated with the use of gabapentin in adults, not seen at an equivalent frequency among placebo-treated patients, were dizziness, somnolence, and peripheral edema.
In the 2 controlled trials in postherpetic neuralgia, 16% of the 336 patients who received gabapentin and 9% of the 227 patients who received placebo discontinued treatment because of an adverse reaction. The adverse reactions that most frequently led to withdrawal in gabapentin-treated patients were dizziness, somnolence, and nausea.
Table 3 lists adverse reactions that occurred in at least 1% of gabapentin-treated patients with postherpetic neuralgia participating in placebo-controlled trials and that were numerically more frequent in the gabapentin group than in the placebo group.
Other reactions in more than 1% of patients but equally or more frequent in the placebo group included pain, tremor, neuralgia, back pain, dyspepsia, dyspnea, and flu syndrome.
There were no clinically important differences between men and women in the types and incidence of adverse reactions. Because there were few patients whose race was reported as other than white, there are insufficient data to support a statement regarding the distribution of adverse reactions by race.
Epilepsy with Partial Onset Seizures (Adjunctive Therapy)
The most common adverse reactions with gabapentin in combination with other antiepileptic drugs in patients greater than 12 years of age, not seen at an equivalent frequency among placebo-treated patients, were somnolence, dizziness, ataxia, fatigue, and nystagmus.
The most common adverse reactions with gabapentin in combination with other antiepileptic drugs in pediatric patients 3 to 12 years of age, not seen at an equal frequency among placebo-treated patients, were viral infection, fever, nausea and/or vomiting, somnolence, and hostility
Approximately 7% of the 2,074 patients greater than 12 years of age and approximately 7% of the 449 pediatric patients 3 to 12 years of age who received gabapentin in premarketing clinical trials discontinued treatment because of an adverse reaction. The adverse reactions most commonly associated with withdrawal in patients greater than 12 years of age were somnolence (1.2%), ataxia (0.8%), fatigue (0.6%), nausea and/or vomiting (0.6%), and dizziness (0.6%). The adverse reactions most commonly associated with withdrawal in pediatric patients were emotional lability (1.6%), hostility (1.3%), and hyperkinesia (1.1%).
Table 4 lists adverse reactions that occurred in at least 1% of gabapentin-treated patients greater than 12 years of age with epilepsy participating in placebo-controlled trials and were numerically more common in the gabapentin group. In these studies, either gabapentin or placebo was added to the patient’s current antiepileptic drug therapy.
Among the adverse reactions occurring at an incidence of at least 10% in gabapentin-treated patients, somnolence and ataxia appeared to exhibit a positive dose-response relationship.
The overall incidence of adverse reactions and the types of adverse reactions seen were similar among men and women treated with gabapentin. The incidence of adverse reactions increased slightly with increasing age in patients treated with either gabapentin or placebo. Because only 3% of patients (28/921) in placebo-controlled studies were identified as nonwhite (black or other), there are insufficient data to support a statement regarding the distribution of adverse reactions by race.
Table 5 lists adverse reactions that occurred in at least 2% of gabapentin-treated patients, age 3 to 12 years of age with epilepsy participating in placebo-controlled trials, and which were numerically more common in the gabapentin group.
Other reactions in more than 2% of pediatric patients 3 to 12 years of age but equally or more frequent in the placebo group included: pharyngitis, upper respiratory infection, headache, rhinitis, convulsions, diarrhea, anorexia, coughing, and otitis media.
4.2Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of gabapentin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary disorders: jaundice
Investigations: elevated creatine kinase, elevated liver function tests
Metabolism and nutrition disorders: hyponatremia
Musculoskeletal and connective tissue disorder: rhabdomyolysis
Nervous system disorders: movement disorder
Psychiatric disorders: agitation
Reproductive system and breast disorders: breast enlargement, changes in libido, ejaculation disorders and anorgasmia
Skin and subcutaneous tissue disorders: angioedema
Adverse reactions following the abrupt discontinuation of gabapentin have also been reported. The most frequently reported reactions were anxiety, insomnia, nausea, pain, and sweating.
5OVERDOSAGE
A lethal dose of gabapentin was not identified in mice and rats receiving single oral doses as high as 8,000 mg/kg. Signs of acute toxicity in animals included ataxia, labored breathing, ptosis, sedation, hypoactivity, or excitation.
Acute oral overdoses of gabapentin up to 49 grams have been reported. In these cases, double vision, slurred speech, drowsiness, lethargy, and diarrhea were observed. All patients recovered with supportive care. Coma, resolving with dialysis, has been reported in patients with chronic renal failure who were treated with gabapentin.
Gabapentin can be removed by hemodialysis. Although hemodialysis has not been performed in the few overdose cases reported, it may be indicated by the patient’s clinical state or in patients with significant renal impairment.
If overexposure occurs, call your poison control center at 1-800-222-1222.
6DESCRIPTION
The active ingredient in gabapentin capsules, USP is gabapentin, USP which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid.
The molecular formula of gabapentin, USP is C
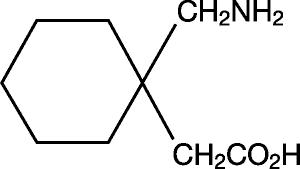
Gabapentin, USP is a white to off-white crystalline solid with a pK
Each gabapentin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin, USP and the following inactive ingredients: black iron oxide, corn starch, D&C Yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C blue #2 aluminum lake, FD&C red #40 aluminum lake, gelatin, mannitol, pharmaceutical glaze, propylene glycol, red iron oxide T3469, silicon dioxide, sodium lauryl sulfate, synthetic black iron oxide, talc, titanium dioxide, and yellow iron oxide T3506.
7HOW SUPPLIED/STORAGE AND HANDLING
Gabapentin capsules, USP are supplied as follows:
100 mg — Each white and light brown capsule printed with
300 mg — Each yellow and light brown capsule printed with
400 mg — Each orange and light brown capsule printed with
Dispense in a tight, light-resistant container as defined in the USP.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( ). Advise the patient to read the FDA-approved patient labeling (
Administration Information
Inform patients that gabapentin is taken orally with or without food.Inform patients that gabapentin is taken orally with or without food.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Prior to initiation of treatment with gabapentin, instruct patients that a rash or other signs or symptoms of hypersensitivity (such as fever or lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a physician immediately . Prior to initiation of treatment with gabapentin, instruct patients that a rash or other signs or symptoms of hypersensitivity (such as fever or lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a physician immediately
Anaphylaxis and Angioedema
Advise patients to discontinue gabapentin and seek medical care if they develop signs or symptoms of anaphylaxis or angioedema [
Dizziness and Somnolence and Effects on Driving and Operating Heavy Machinery
Advise patients that gabapentin may cause dizziness, somnolence, and other symptoms and signs of CNS depression. Other drugs with sedative properties may increase these symptoms. Accordingly, although patients’ ability to determine their level of impairment can be unreliable, advise them neither to drive a car nor to operate other complex machinery until they have gained sufficient experience on gabapentin to gauge whether or not it affects their mental and/or motor performance adversely. Inform patients that it is not known how long this effect lasts . Advise patients that gabapentin may cause dizziness, somnolence, and other symptoms and signs of CNS depression. Other drugs with sedative properties may increase these symptoms. Accordingly, although patients’ ability to determine their level of impairment can be unreliable, advise them neither to drive a car nor to operate other complex machinery until they have gained sufficient experience on gabapentin to gauge whether or not it affects their mental and/or motor performance adversely. Inform patients that it is not known how long this effect lasts
Suicidal Thinking and Behavior
Counsel the patient, their caregivers, and families that AEDs, including gabapentin, may increase the risk of suicidal thoughts and behavior. Advise patients of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients to report behaviors of concern immediately to healthcare providers . Counsel the patient, their caregivers, and families that AEDs, including gabapentin, may increase the risk of suicidal thoughts and behavior. Advise patients of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients to report behaviors of concern immediately to healthcare providers
Use in Pregnancy
Instruct patients to notify their physician if they become pregnant or intend to become pregnant during therapy, and to notify their physician if they are breastfeeding or intend to breastfeed during therapy . Instruct patients to notify their physician if they become pregnant or intend to become pregnant during therapy, and to notify their physician if they are breastfeeding or intend to breastfeed during therapy
Encourage patients to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 . Encourage patients to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334
Brands listed are the trademarks of their respective owners.Brands listed are the trademarks of their respective owners.
Manufactured by: Watson Pharma Private Limited Verna, Salcette Goa 403 722 INDIA Manufactured by:
Distributed by: Actavis Pharma, Inc. Parsippany, NJ 07054 USA Distributed by:
40-923440-9234
Revised — December 2017Revised — December 2017
9MEDICATION GUIDE
Gabapentin
What is the most important information I should know about gabapentin capsules?
Do not stop taking gabapentin capsules without first talking to your healthcare provider.
Stopping gabapentin capsules suddenly can cause serious problems.
Gabapentin can cause serious side effects including:
1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop taking gabapentin capsules without first talking to a healthcare provider.
- Stopping gabapentin capsules suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
2. Changes in behavior and thinking - Using gabapentin capsules in children 3 to 12 years of age can cause emotional changes, aggressive behavior, problems with concentration, restlessness, changes in school performance, and hyperactivity.
3. Gabapentin may cause serious or life-threatening allergic reactions that may affect your skin or other parts of your body such as your liver or blood cells. This may cause you to be hospitalized or to stop gabapentin. You may or may not have a rash with an allergic reaction caused by gabapentin. Call a healthcare provider right away if you have any of the following symptoms:
- skin rash
- hives
- difficulty breathing
- fever
- swollen glands that do not go away
- swelling of your face, lips, throat, or tongue
- yellowing of your skin or of the whites of the eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- unexpected muscle pain
- frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking gabapentin capsules.
What are gabapentin capsules?
Gabapentin capsules are a prescription medicine used to treat:
- Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults.
- Partial seizures when taken together with other medicines in adults and children 3 years of age and older with seizures.
Who should not take gabapentin capsules?
Do not take gabapentin capsules if you are allergic to gabapentin or any of the other ingredients in gabapentin capsules. See the end of this Medication Guide for a complete list of ingredients in gabapentin capsules.
What should I tell my healthcare provider before taking gabapentin capsules?
Before taking gabapentin capsules, tell your healthcare provider if you:
- have or have had kidney problems or are on hemodialysis
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have diabetes
- are pregnant or plan to become pregnant. It is not known if gabapentin can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking gabapentin capsules. You and your healthcare provider will decide if you should take gabapentin capsules while you are pregnant.
- are breastfeeding or plan to breastfeed. Gabapentin can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take gabapentin capsules.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking gabapentin capsules with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take gabapentin capsules?
- Take gabapentin capsules exactly as prescribed. Your healthcare provider will tell you how much gabapentin capsules to take.
If you take too many gabapentin capsules, call your healthcare provider or your local Poison Control Center right away at 1-800-222-1222.
What should I avoid while taking gabapentin capsules?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking gabapentin capsules without first talking with your healthcare provider. Taking gabapentin capsules with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin capsules affect you. Gabapentin can slow your thinking and motor skills.
What are the possible side effects of gabapentin?
Gabapentin may cause serious side effects including:
See “What is the most important information I should know about gabapentin capsules?”
- problems driving while using gabapentin. See “What I should avoid while taking gabapentin capsules?”
- sleepiness and dizziness, which could increase the occurrence of accidental injury, including falls
- The most common side effects of gabapentin include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of gabapentin. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store gabapentin capsules?
- Store gabapentin capsules between 68°F to 77°F (20°C to 25°C).
Keep gabapentin capsules and all medicines out of the reach of children.
General information about the safe and effective use of gabapentin capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use gabapentin capsules for a condition for which it was not prescribed. Do not give gabapentin capsules to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about gabapentin capsules. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about gabapentin capsules that was written for healthcare professionals.
For medical inquiries or to report side effects regarding gabapentin capsules, please call Actavis at 1-800-432-8534.
What are the ingredients in gabapentin capsules, USP?
Active ingredient: gabapentin, USP
Inactive ingredients in the capsules: black iron oxide, corn starch, D&C Yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C blue #2 aluminum lake, FD&C red #40 aluminum lake, gelatin, mannitol, pharmaceutical glaze, propylene glycol, red iron oxide T3469, silicon dioxide, sodium lauryl sulfate, synthetic black iron oxide, talc, titanium dioxide, and yellow iron oxide T3506.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Distributed by:
40-9234
(MG 41-1205/1217)
10PharmapureRx ®Pill Swallowing Spray™

11Gaba 300-EZS
