Brand Name
Brovana
Generic Name
Arformoterol
View Brand Information FDA approval date: May 31, 2021
Classification: beta2-Adrenergic Agonist
Form: Solution
What is Brovana (Arformoterol)?
BROVANA Inhalation Solution is a long-acting beta 2 -adrenergic agonist indicated for: Long-term, twice daily administration in the maintenance treatment of broncho-constriction in patients with chronic obstructive pulmonary disease , including chronic bronchitis and emphysema.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
BROVANA (Arformoterol Tartrate)
1DOSAGE AND ADMINISTRATION
The recommended dose of BROVANA (arformoterol tartrate) Inhalation Solution is one 15 mcg unit-dose vial administered twice daily (morning and evening) by nebulization. A total daily dose of greater than 30 mcg (15 mcg twice daily) is not recommended.
BROVANA Inhalation Solution should be administered by the orally inhaled route via a standard jet nebulizer connected to an air compressor (see the accompanying
If the recommended maintenance treatment regimen fails to provide the usual response, medical advice should be sought immediately, as this is often a sign of destabilization of COPD. Under these circumstances, the therapeutic regimen should be reevaluated and additional therapeutic options should be considered.
No dose adjustment is required for patients with renal or hepatic impairment. However, since the clearance of BROVANA Inhalation Solution is prolonged in patients with hepatic impairment, they should be monitored closely.
The drug compatibility (physical and chemical), efficacy, and safety of BROVANA Inhalation Solution when mixed with other drugs in a nebulizer have not been established.
The safety and efficacy of BROVANA Inhalation Solution have been established in clinical trials when administered using the PARI LC
2DOSAGE FORMS AND STRENGTHS
BROVANA (arformoterol tartrate) Inhalation Solution is supplied as a sterile solution for nebulization in low-density polyethylene unit-dose vials. Each 2 mL vial contains 15 mcg of arformoterol equivalent to 22 mcg of arformoterol tartrate.
3CONTRAINDICATIONS
BROVANA Inhalation Solution is contraindicated in patients with a history of hypersensitivity to arformoterol, racemic formoterol or to any other components of this product.
Use of a LABA, including BROVANA Inhalation Solution, without an inhaled cortisteroid is contraindicated in patients with asthma [
4ADVERSE REACTIONS
Long-acting beta
4.1Beta2-Agonist Adverse Reaction Profile
Adverse reactions to BROVANA Inhalation Solution are expected to be similar in nature to other beta
4.2Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adults with COPD in Short-Term Trials (12 weeks)
The safety data described below for adults ≥35 years of age are based on 2 clinical trials of 12 weeks. In the 2 trials of 12 weeks duration, 1456 patients (860 males and 596 females, ages 34 to 89 years old) with COPD were treated with BROVANA Inhalation Solution 15 mcg twice daily, 25 mcg twice daily, 50 mcg once daily, salmeterol 42 mcg twice daily, or placebo. The racial/ethnic distribution in these two trials included 1383 Caucasians, 49 Blacks, 10 Asians, and 10 Hispanics, and 4 patients classified as Other.
Among the 1,456 COPD patients in two 12-week, placebo-controlled trials, 288 were treated with BROVANA Inhalation Solution 15 mcg twice daily and 293 were treated with placebo. Doses of 25 mcg twice daily and 50 mcg once daily were also evaluated.
Table 1 shows adverse reaction rates among patients from these two trials where the frequency was greater than or equal to 2% in the BROVANA Inhalation Solution 15 mcg twice daily group and where the rate in the BROVANA Inhalation Solution 15 mcg twice daily group exceeded the rate in the placebo group. The total number and percent of patients who reported adverse events were 202 (70%) in the 15 mcg twice daily and 219 (75%) in the placebo groups. Ten adverse events demonstrated a dose relationship: asthenia, fever, bronchitis, COPD, headache, vomiting, hyperkalemia, leukocytosis, nervousness, and tremor.
Adverse events occurring in patients treated with BROVANA Inhalation Solution 15 mcg twice daily with a frequency of <2%, but greater than placebo, were as follows:
Body as a Whole: abscess, allergic reaction, digitalis intoxication, fever, hernia, injection site pain, neck rigidity, neoplasm, pelvic pain, retroperitoneal hemorrhage
Cardiovascular: arteriosclerosis, atrial flutter, AV block, congestive heart failure, heart block, myocardial infarct, QT interval prolonged, supraventricular tachycardia, inverted T-wave
Digestive: constipation, gastritis, melena, oral moniliasis, periodontal abscess, rectal hemorrhage
Metabolic and Nutritional Disorders: dehydration, edema, glucose tolerance decreased, gout, hyperglycemia, hyperlipemia, hypoglycemia, hypokalemia
Musculoskeletal: arthralgia, arthritis, bone disorder, rheumatoid arthritis, tendinous contracture
Nervous: agitation, cerebral infarct, circumoral paresthesia, hypokinesia, paralysis, somnolence, tremor
Respiratory: carcinoma of the lung, respiratory disorder, voice alteration
Skin and Appendages: dry skin, herpes simplex, herpes zoster, skin discoloration, skin hypertrophy
Special Senses: abnormal vision, glaucoma
Urogenital: breast neoplasm, calcium crystalluria, cystitis, glycosuria, hematuria, kidney calculus, nocturia, PSA increase, pyuria, urinary tract disorder, urine abnormality.
In these trials, the overall frequency of all cardiovascular adverse events was 6.9% in BROVANA Inhalation Solution 15 mcg twice daily and 13.3% in the placebo group. There were no frequently occurring specific cardiovascular adverse events for BROVANA Inhalation Solution (frequency ≥1% and greater than placebo). The rate of COPD exacerbations was also comparable between the BROVANA Inhalation Solution 15 mcg twice daily and placebo groups, 12.2% and 15.1%, respectively.
Adults with COPD in Long-Term (52-week) Safety Trial
BROVANA Inhalation Solution was evaluated in one 52 week double-blind, randomized, placebo-controlled, safety trial conducted in patients with moderate to severe COPD. The primary endpoint was time to either respiratory death or first COPD exacerbation-related hospitalization, whichever occurred first. The event had to be a death or hospitalization for which the patient's respiratory status was predominant and/or inciting contributor, as determined by the clinical investigator. The objective of the trial was to demonstrate that the risk of respiratory death or COPD exacerbation-related hospitalization for patients treated with BROVANA Inhalation Solution was not greater than 40% more than the risk for patient treated with placebo. A total of 841 patients (479 males and 361 females, ages 41 to 94 years old) with COPD were randomized: 420 to BROVANA Inhalation Solution 15 mcg twice daily and 421 to placebo. Of the randomized patients, 255 (61%) in the BROVANA Inhalation Solution group and 211 (50%) in the placebo group, completed one year of treatment. The trial objective was met demonstrating that COPD patients treated with BROVANA Inhalation Solution are not at an increased risk of respiratory death or COPD exacerbation-related hospitalizations compared to placebo.
5DRUG ABUSE AND DEPENDENCE
There were no reported cases of abuse or evidence of drug dependence with the use of BROVANA Inhalation Solution in the clinical trials.
6OVERDOSAGE
The expected signs and symptoms associated with overdosage of BROVANA (arformoterol tartrate) Inhalation Solution are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms listed under
Treatment of overdosage consists of discontinuation of BROVANA Inhalation Solution together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of BROVANA Inhalation Solution. Cardiac monitoring is recommended in cases of overdosage.
7DESCRIPTION
BROVANA (arformoterol tartrate) Inhalation Solution is a sterile, clear, colorless, aqueous solution of the tartrate salt of arformoterol, the (R,R)-enantiomer of formoterol.
Arformoterol is a selective beta2-adrenergic bronchodilator. The chemical name for arformoterol tartrate is formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, (2R,3R)-2,3-dihydroxybutanedioate (1:1 salt), and its established structural formula is as follows:
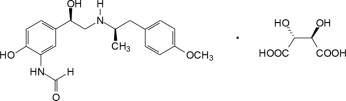
The molecular weight of
Arformoterol tartrate is the United States Adopted Name (USAN) for (R,R)-formoterol L-tartrate.
BROVANA (arformoterol tartrate) Inhalation Solution is supplied as 2 mL of arformoterol tartrate solution packaged in 2.1 mL unit-dose, low-density polyethylene (LDPE) unit-dose vials. Each unit-dose vial contains 15 mcg of arformoterol (equivalent to 22 mcg of arformoterol tartrate) in a sterile, isotonic saline solution, pH-adjusted to 5.0 with citric acid and sodium citrate.
BROVANA Inhalation Solution requires no dilution before administration by nebulization. Like all other nebulized treatments, the amount delivered to the lungs will depend upon patient factors, the nebulizer used, and compressor performance. Using the PARI LC® Plus nebulizer (with mouthpiece) connected to a PARI DURA NEB™ 3000 compressor under
Patients should be carefully instructed on the correct use of this drug product (please refer to the accompanying
8HOW SUPPLIED/STORAGE AND HANDLING
BROVANA (arformoterol tartrate) Inhalation Solution is supplied in a single strength (15 mcg of arformoterol, equivalent to 22 mcg of arformoterol tartrate) as 2 mL of a sterile solution in low-density polyethylene (LDPE) unit-dose vials overwrapped in foil. BROVANA Inhalation Solution is available in a shelf-carton containing 30 or 60 unit-dose vials.
NDC 27437-060-30: carton of 30 individually pouched unit-dose vials.
NDC 27437-060-60: carton of 60 individually pouched unit-dose vials.
Storage and Handling
Store BROVANA Inhalation Solution in the protective foil pouch under refrigeration at 36°-46°F (2°-8°C). Protect from light and excessive heat. An opened unit-dose vial should be used right away. Discard any unit-dose vial if the solution is not colorless. Unopened foil pouches of BROVANA Inhalation Solution can also be stored at room temperature 68°-77°F (20°-25°C) for up to 6 weeks. If stored at room temperature, discard if not used after 6 weeks or if past the expiration date, whichever is sooner.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use) with each new prescription and refill.
The complete text of the Patient Information is reprinted at the end of this document. Patients should be given the following information:
Serious Asthma-Related Events, Acute Exacerbations or Deteriorations
Patients should be informed that long-acting beta
BROVANA Inhalation Solution is not indicated to relieve acute respiratory symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta
Appropriate Dosing
Patients should not stop using BROVANA Inhalation Solution unless told to do so by a healthcare provider because symptoms may get worse. Patients should not inhale more than one dose at any one time. The daily dosage of BROVANA Inhalation Solution should not exceed one unit-dose vial (15 mcg) by inhalation twice daily (30 mcg total daily dose). Excessive use of sympathomimetics may cause significant cardiovascular effects, and may be fatal.
Concomitant Therapy
Patients who have been taking inhaled, short-acting beta
BROVANA Inhalation Solution should not be used in conjunction with other inhaled medications containing long-acting beta
Common Adverse Reactions with Beta
Patients should be informed that treatment with beta
Instructions for Administration
It is important that patients understand how to use BROVANA Inhalation Solution with a nebulizer appropriately and how it should be used in relation to other medications to treat COPD they are taking
Women should be advised to contact their physician if they become pregnant or if they are nursing.
FDA-Approved Patient Information
See the accompanying
10PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
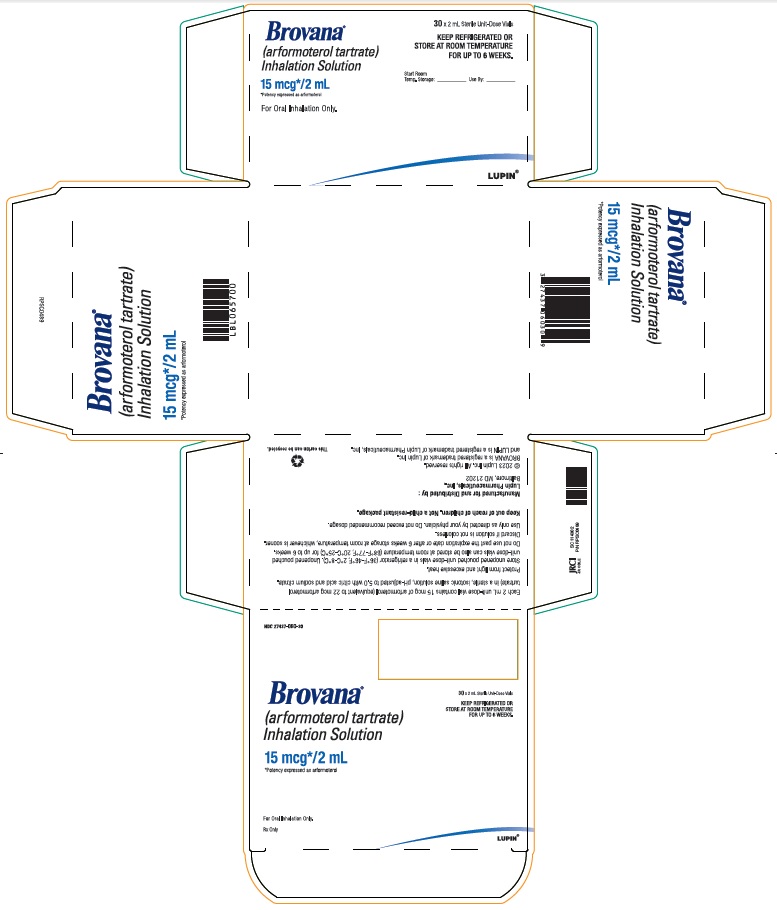
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 60 VIAL CARTON
NDC 27437-060-60
60x 2mL Sterile Unit-Dose Vials
KEEP REFRIGERATED OR STORE AT ROOM TEMPERATURE
FOR UP TO 6 WEEKS.
Brovana
(arformoterol tartrate)
Inhalation Solution
15 mcg* / 2 mL
* Potency expressed as arformoterol
For Oral Inhalation Only.
Rx Only

PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 30 VIAL CARTON
NDC 27437-060-30
30x 2mL Sterile Unit-Dose Vials
KEEP REFRIGERATED OR STORE AT ROOM TEMPERATURE
FOR UP TO 6 WEEKS.
Brovana
(arformoterol tartrate)
Inhalation Solution
15 mcg* / 2 mL
* Potency expressed as arformoterol
For Oral Inhalation Only.
Rx Only
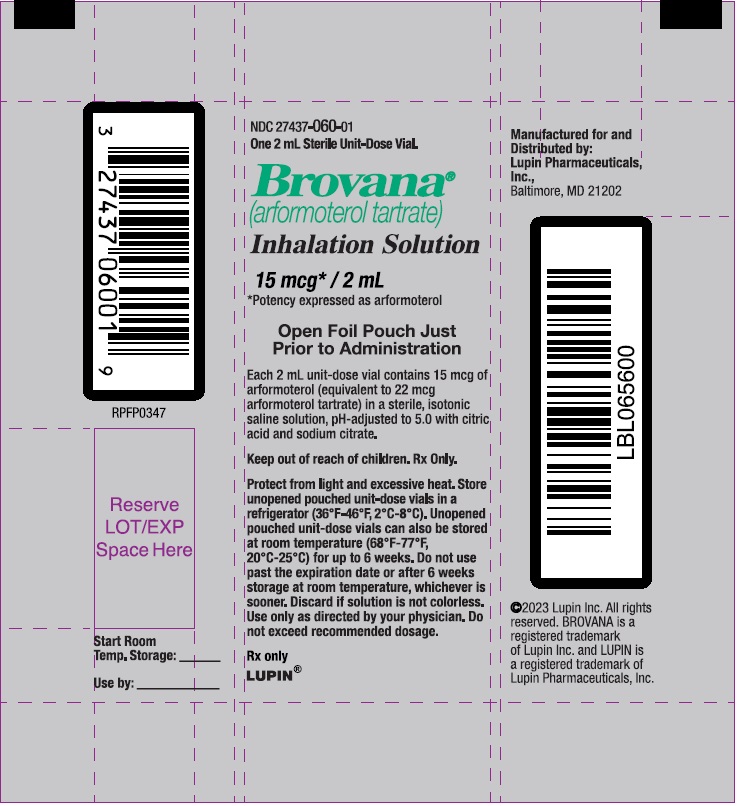
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Single VIAL POUCH
NDC 27437-060-01
One 2 mL Sterile Unit-Dose Vial.
Brovana
(arformoterol tartrate)
Inhalation Solution
15 mcg* / 2 mL
* Potency expressed as arformoterol
Open Foil Pouch Just Prior to Administration
Each 2 mL unit-dose vial contains 15mcg of arformoterol (equivalent to 22 mcg arformoterol tartrate) in a sterile, isotonic saline solution, pH- adjusted to 5.0 with citric acid and sodium citrate.
Keep out of reach of children. Rx Only.
Protect from light and excessive heat. Store unopened pouched unit-dose vials in a refrigerator (36ºF-46ºF, 2ºC-8ºC). Unopened pouched unit-dose vials can also be stored at room temperature (68ºF-77ºF, 20ºC-25ºC) for up to 6 weeks. Do not use past the expiration date or after 6 weeks storage at room temperature, whichever is sooner. Discard if solution is not colorless. Use only as directed by your physician. Do not exceed recommended dosage.