Motegrity
What is Motegrity (Prucalopride)?
Living with chronic constipation can be uncomfortable and frustrating, affecting everything from daily energy levels to overall quality of life. For many people, dietary changes and over-the-counter laxatives aren’t enough to bring lasting relief. That’s where Motegrity (prucalopride) can make a difference.
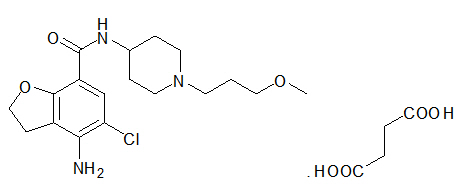
Motegrity is a prescription medication approved by the U.S. Food and Drug Administration (FDA) to treat chronic idiopathic constipation (CIC) in adults. It belongs to a class of drugs known as serotonin-4 (5-HT4) receptor agonists, which work differently from most other laxatives by directly stimulating the natural movement of the intestines.
Approved in 2018, Motegrity is a newer, targeted therapy designed to help people who haven’t found sufficient relief from other treatments. When used as prescribed, it can help restore a more regular, comfortable bowel pattern and reduce the distress associated with persistent constipation.
What does Motegrity do?
Motegrity is used to treat chronic idiopathic constipation (CIC), a long-term condition where bowel movements are infrequent or difficult to pass without an identifiable cause such as a medical illness or medication.
Unlike occasional constipation that may be triggered by diet, stress or travel, chronic idiopathic constipation often persists for months or even years. Patients may experience symptoms like bloating, abdominal discomfort and the sensation of incomplete evacuation.
Motegrity helps increase the number of complete bowel movements per week, making bowel activity more predictable and reducing associated discomfort.
In clinical trials, patients taking Motegrity experienced significantly more spontaneous bowel movements compared to those taking a placebo (FDA, 2018). Many users reported improvement within the first few weeks of treatment, along with less straining and abdominal bloating.
For individuals whose constipation doesn’t respond well to fiber, stool softeners or traditional laxatives, Motegrity offers a novel approach to restoring gut motility naturally.
How does Motegrity work?
Motegrity works by activating serotonin-4 (5-HT4) receptors located in the gastrointestinal (GI) tract. These receptors help coordinate the movement of the intestines, a process known as peristalsis, which propels stool through the colon.
By stimulating these receptors, prucalopride enhances the muscle contractions in the colon, helping the bowel move more efficiently. This mechanism targets the root cause of chronic constipation, sluggish intestinal movement rather than just softening stool like most laxatives do.
Clinically, this mechanism helps promote a more natural bowel rhythm. Because Motegrity acts specifically on the gut’s serotonin system, it helps improve motility without significantly affecting the brain or other organs.
Healthcare providers often recommend Motegrity when standard treatments such as osmotic or stimulant laxatives fail to bring adequate relief. It’s typically used as a long-term therapy for adults who require consistent management of chronic constipation.
Motegrity side effects
While Motegrity is generally well tolerated, some people may experience side effects. Most are mild and tend to lessen over time as the body adjusts to the medication.
Common side effects include:
- Headache
- Abdominal pain
- Nausea
- Diarrhea
- Dizziness
Less common but possible side effects:
- Vomiting
- Fatigue
- Changes in mood or sleep
Serious side effects are rare, but patients should be aware of them. In clinical studies, a small number of people experienced mood changes or depressive symptoms, including rare reports of suicidal thoughts. Anyone experiencing such symptoms should contact a healthcare provider right away.
Motegrity should not be used by individuals with:
- A history of intestinal perforation or obstruction
- Inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis
- Severe kidney disease unless advised and monitored by a physician
Patients should also seek immediate medical attention if they experience signs of an allergic reaction, such as rash, swelling or difficulty breathing.
When used under medical supervision, Motegrity offers a safe and effective solution for patients struggling with long-term constipation.
Motegrity dosage
Motegrity comes in tablet form, taken once daily by mouth, with or without food. It’s designed for consistent, daily use to maintain regular bowel movements.
Doctors typically start patients on a standardized daily dose, adjusting if necessary based on how the body responds or if kidney function is impaired.
Because Motegrity affects the serotonin system in the gut, monitoring may include:
- Evaluating mental health and mood, especially during the first few weeks
- Checking kidney function, as dose adjustments may be needed in severe kidney disease
- Tracking bowel frequency and comfort, to ensure optimal results
Patients should continue any recommended hydration and dietary fiber habits while taking Motegrity, as these can enhance its effectiveness. If no improvement is seen after several weeks, doctors may reassess whether the medication remains appropriate.
Does Motegrity have a generic version?
As of 2025, no FDA-approved generic version of Motegrity (prucalopride) is available in the United States. The drug remains available only as the brand-name formulation manufactured by Takeda Pharmaceuticals.
However, prucalopride may be available under different brand names in some international markets, such as Resolor, particularly in Europe and Canada.
When the patent expires, generic versions will be required to demonstrate the same safety, efficacy, and quality as Motegrity before approval. Until then, patients can discuss with their healthcare providers or pharmacists about cost-assistance programs or insurance coverage options for the brand-name medication.
Conclusion
Motegrity (prucalopride) is an innovative prescription medication that helps patients with chronic idiopathic constipation achieve more regular, comfortable bowel movements. By targeting serotonin receptors in the gut, it works in harmony with the body’s natural digestive rhythm rather than relying on harsh laxative effects.
For adults struggling with persistent constipation that hasn’t responded to lifestyle changes or over-the-counter remedies, Motegrity offers a scientifically advanced and patient-friendly option. When prescribed and monitored by a qualified healthcare professional, it can play an important role in restoring digestive comfort and improving quality of life.
References
- U.S. Food and Drug Administration (FDA). (2018). Motegrity (prucalopride) https://www.fda.gov/
- Mayo Clinic. (2024). Prucalopride (Oral Route) https://www.mayoclinic.org/
- MedlinePlus. (2024). Prucalopride: Drug Information. https://www.medline.com/
- National Institutes of Health (NIH). (2023). Chronic Constipation: Treatment and Management. https://www.nih.gov/
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- 1 mg prucalopride: White to off-white, round, biconvex film-coated tablet debossed with "PRU 1" on one side and no debossing on the other side.
- 2 mg prucalopride: Pink, round, biconvex film-coated tablet debossed with "PRU 2" on one side and no debossing on the other side.
- A history of hypersensitivity to MOTEGRITY. Reactions including dyspnea, rash, pruritus, urticaria, and facial edema have been observed
- Intestinal perforation or obstruction due to structural or functional disorder of the gut wall, obstructive ileus, severe inflammatory conditions of the intestinal tract such as Crohn's disease, ulcerative colitis, and toxic megacolon/megarectum.
- Lumpy or hard stools
- Sensation of incomplete evacuation
- Straining at defecation
- NDC 54092-546-01: HDPE bottle of 30 tablets, with child-resistant closure.
- NDC 54092-547-01: HDPE bottle of 30 tablets, with child-resistant closure.
- Suicidal Ideation and Behavior: Inform patients, their caregivers, and family members that suicidal ideation and behavior, self-injurious ideation as well as new onset or worsening depression have been reported in patients treated with MOTEGRITY. Advise them to be aware of any unusual changes in mood or behavior, new onset or worsening of depression, or the emergence of suicidal thoughts or behavior. Instruct patients, caregivers, and family members to discontinue MOTEGRITY immediately and contact their healthcare provider if any of these symptoms occur [see .
- Pregnancy: Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to MOTEGRITY during pregnancy

