Generic Name
Latanoprost
Brand Names
Xalatan, Xelpros, Iyuzeh
FDA approval date: March 20, 1995
Classification: Prostaglandin Analog
Form: Solution
What is Xalatan (Latanoprost)?
XELPROS
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
XALATAN (Latanoprost)
1INDICATIONS AND USAGE
XALATAN is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose as normal.
The dosage of XALATAN should not exceed once daily; the combined use of two or more prostaglandins, or prostaglandin analogs including XALATAN is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Reduction of the IOP starts approximately 3 to 4 hours after administration and the maximum effect is reached after 8 to 12 hours.
XALATAN may be used concomitantly with other topical ophthalmic drug products to lower IOP.
3DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing latanoprost 50 mcg/mL (0.005%).
4CONTRAINDICATIONS
Known hypersensitivity to latanoprost, benzalkonium chloride, or any other ingredients in this product.
5ADVERSE REACTIONS
The following adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the label:
- Iris pigmentation changes
- Eyelid skin darkening
- Eyelash changes (increased length, thickness, pigmentation, and number of lashes)
- Intraocular inflammation (iritis/uveitis)
- Macular edema, including cystoid macular edema
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
XALATAN was studied in three multicenter, randomized, controlled clinical trials. Patients received 50 mcg/mL XALATAN once daily or 5 mg/mL active-comparator (timolol) twice daily. The patient population studied had a mean age of 65±10 years. Seven percent of patients withdrew before the 6-month endpoint.
Less than 1% of the patients treated with XALATAN required discontinuation of therapy because of intolerance to conjunctival hyperemia.
5.2Postmarketing Experience
The following reactions have been identified during postmarketing use of XALATAN in clinical practice. Because they are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to XALATAN, or a combination of these factors, include:
Nervous System Disorders: Dizziness; headache; toxic epidermal necrolysis
Eye Disorders: Eyelash and vellus hair changes of the eyelid (increased length, thickness, pigmentation, and number of eyelashes); keratitis; corneal edema and erosions; intraocular inflammation (iritis/uveitis); macular edema, including cystoid macular edema; trichiasis; periorbital and lid changes resulting in deepening of the eyelid sulcus; iris cyst; eyelid skin darkening; localized skin reaction on the eyelids; conjunctivitis; pseudopemphigoid of the ocular conjunctiva.
Respiratory, Thoracic and Mediastinal Disorders: Asthma and exacerbation of asthma; dyspnea
Gastrointestinal Disorders: Nausea; vomiting
Skin and Subcutaneous Tissue Disorders: Pruritis
Infections and Infestations: Herpes keratitis
Cardiac Disorders: Angina; palpitations; angina unstable
General Disorders and Administration Site Conditions: Chest pain
6OVERDOSAGE
IV infusion of up to 3 mcg/kg of latanoprost in healthy volunteers produced mean plasma concentrations 200 times higher than during clinical treatment with XALATAN and no adverse reactions were observed. IV dosages of 5.5 to 10 mcg/kg caused abdominal pain, dizziness, fatigue, hot flushes, nausea, and sweating.
If overdosage with XALATAN occurs, treatment should be symptomatic.
7DESCRIPTION
Latanoprost is a prostaglandin F
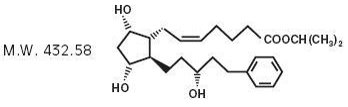
Latanoprost is a colorless to slightly yellow oil that is very soluble in acetonitrile and freely soluble in acetone, ethanol, ethyl acetate, isopropanol, methanol, and octanol. It is practically insoluble in water.
XALATAN (latanoprost ophthalmic solution) 0.005% is supplied as a sterile, isotonic, buffered aqueous solution of latanoprost with a pH of approximately 6.7 and an osmolality of approximately 267 mOsmol/kg. Each mL of XALATAN contains 50 mcg of latanoprost. Benzalkonium chloride, 0.02% is added as a preservative. The inactive ingredients are disodium phosphate anhydrous, sodium chloride, sodium dihydrogen phosphate monohydrate, and water for injection. One drop contains approximately 1.5 mcg of latanoprost.
8HOW SUPPLIED/STORAGE AND HANDLING
XALATAN is a clear, isotonic, buffered, preserved colorless solution of latanoprost 50 mcg/mL (0.005%). It is supplied as a 2.5 mL solution in a 5 mL clear low density polyethylene bottle with a clear polyethylene dropper tip, a turquoise high density polyethylene screw cap, and a tamper-evident clear low density polyethylene overcap.
2.5 mL fill, 50 mcg/mL (0.005%): Package of 1 bottle: NDC 58151-419-35
Storage: Protect from light. Store unopened bottle(s) under refrigeration at 2°C to 8°C (36°F to 46°F). During shipment to the patient, the bottle may be maintained at temperatures up to 40°C (104°F) for a period not exceeding 8 days. Once a bottle is opened for use, it may be stored at room temperature up to 25°C (77°F) for 6 weeks.
9PATIENT COUNSELING INFORMATION
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of XALATAN
Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with XALATAN. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of the multiple-dose container.
Contact Lens Use
Advise patients that XALATAN contains benzalkonium chloride, which may be absorbed by contact lenses. Contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of XALATAN.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
If a Dose is Missed
Advise patients that if one dose is missed, treatment should continue with the next dose as normal.
Distributed by:
Made in Belgium
© 2023 Viatris Inc.
XALATAN is a registered trademark of Pfizer PFE Holdings 4 LLC, a Viatris Company.
UPJ:XLTN:R1
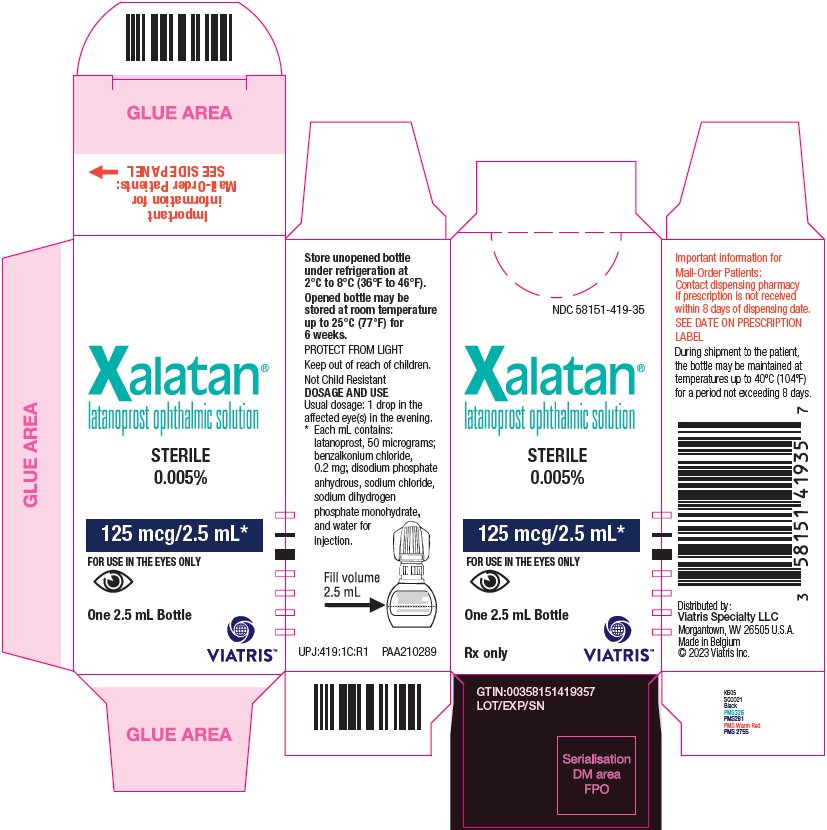
10PRINCIPAL DISPLAY PANEL – 0.005%
NDC 58151-419-35
Xalatan
125 mcg/2.5 mL*
FOR USE IN THE EYES ONLY
One 2.5 mL Bottle
Rx only
Store unopened bottle
Opened bottle may be
PROTECT FROM LIGHT
Keep out of reach of children.
Not Child Resistant
DOSAGE AND USE
Usual dosage: 1 drop in the
affected eye(s) in the evening.
Usual dosage: 1 drop in the
affected eye(s) in the evening.
* Each mL contains:
UPJ:419:1C:R1 PAA210289
Important information for
SEE DATE ON PRESCRIPTION
During shipment to the patient,
Distributed by:
Made in Belgium
© 2023 Viatris Inc.