Brand Name
Azilect
Generic Name
Rasagiline
View Brand Information FDA approval date: July 10, 2006
Classification: Monoamine Oxidase Inhibitor
Form: Tablet
What is Azilect (Rasagiline)?
AZILECT is indicated for the treatment of Parkinson’s disease . AZILECT, a monoamine oxidase -B inhibitor , is indicated for the treatment of Parkinson’s disease
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Azilect (Rasagiline mesylate)
1INDICATIONS AND USAGE
AZILECT (rasagiline tablets) is indicated for the treatment of Parkinson’s disease (PD).
2DOSAGE FORMS AND STRENGTHS
AZILECT 0.5 mg Tablets: White to off-white, round, flat, beveled tablets, debossed with “GIL 0.5” on one side and plain on the other side.
AZILECT 1 mg Tablets: White to off-white, round, flat, beveled tablets, debossed with “GIL 1” on one side and plain on the other side.
3CONTRAINDICATIONS
AZILECT is contraindicated for use with meperidine, tramadol, methadone, propoxyphene, and MAO inhibitors (MAOIs), including other selective MAO-B inhibitors, because of risk of serotonin syndrome
AZILECT is contraindicated for use with St. John’s wort and with cyclobenzaprine.
AZILECT is contraindicated for use with dextromethorphan because of risk of episode of psychosis or bizarre behavior.
4ADVERSE REACTIONS
The following adverse reactions are described in more detail in the
- Hypertension
- Serotonin Syndrome
- Falling Asleep During Activities of Daily Living and Somnolence
- Hypotension / Orthostatic Hypotension
- Dyskinesia
- Hallucinations / Psychotic-Like Behavior
- Impulse Control /Compulsive Behaviors
- Withdrawal-Emergent Hyperpyrexia and Confusion
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the incidence of adverse reactions in the clinical trials of another drug and may not reflect the rates of adverse reactions observed in practice.
During the clinical development of AZILECT, Parkinson’s disease patients received AZILECT as initial monotherapy (Study 1) and as adjunct therapy (Study 2, Study 3, Study 4). As the populations in these studies differ, not only in the adjunct use of dopamine agonists or levodopa during AZILECT treatment, but also in the severity and duration of their disease, the adverse reactions are presented separately for each study.
Monotherapy Use of AZILECT
In Study 1, approximately 5% of the 149 patients treated with AZILECT discontinued treatment due to adverse reactions compared to 2% of the 151 patients who received placebo.
The only adverse reaction that led to the discontinuation of more than one patient was hallucinations.
The most commonly observed adverse reactions in Study 1 (incidence in AZILECT-treated patients 3% or greater than the incidence in placebo-treated patients) included flu syndrome, arthralgia, depression, and dyspepsia. Table 1 lists adverse reactions that occurred in 2% or greater of patients receiving AZILECT as monotherapy and were numerically more frequent than in the placebo group in Study 1.
*Incidence 2% or greater in AZILECT 1 mg group and numerically more frequent than in placebo group
There were no significant differences in the safety profile based on age or gender.
Adjunct Use of AZILECT
AZILECT was studied as an adjunct therapy without levodopa (Study 2), or as an adjunct therapy to levodopa, with some patients also taking dopamine agonists, COMT inhibitors, anticholinergics, or amantadine (Study 3 and Study 4).
In Study 2, approximately 8% of the 162 patients treated with AZILECT discontinued treatment due to adverse reactions compared to 4% of the 164 patients who received placebo.
Adverse reactions that led to the discontinuation of more than one patient were nausea and dizziness.
The most commonly observed adverse reactions in Study 2 (incidence in AZILECT-treated patients 3% or greater than incidence in placebo-treated patients) included peripheral edema, fall, arthralgia, cough, and insomnia. Table 2 lists adverse reactions that occurred in 2% or greater in patients receiving AZILECT as adjunct therapy without levodopa and numerically more frequent than in the placebo group in Study 2.
*Incidence 2% or greater in AZILECT 1 mg group and numerically more frequent than in placebo group
There were no significant differences in the safety profile based on age or gender.
In Study 3, adverse event reporting was considered more reliable than Study 4; therefore, only the adverse event data from Study 3 are presented below.
In Study 3, approximately 9% of the 164 patients treated with AZILECT 0.5 mg/day and 7% of the 149 patients treated with AZILECT 1 mg/day discontinued treatment due to adverse reactions, compared to 6% of the 159 patients who received placebo. The adverse reactions that led to discontinuation of more than one AZILECT-treated patient were diarrhea, weight loss, hallucination, and rash.
The most commonly observed adverse reactions in Study 3 (incidence in AZILECT-treated patients 3% or greater than the incidence in placebo-treated patients) included dyskinesia, accidental injury, weight loss, postural hypotension, vomiting, anorexia, arthralgia, abdominal pain, nausea, constipation, dry mouth, rash, abnormal dreams, fall, and tenosynovitis.
Table 3 lists adverse reactions that occurred in 2% or greater of patients treated with AZILECT 1 mg/day and that were numerically more frequent than the placebo group in Study 3.
*Incidence 2% or greater in AZILECT 1 mg group and numerically more frequent than in placebo group
Several of the more common adverse reactions seemed dose-related, including weight loss, postural hypotension, and dry mouth.
There were no significant differences in the safety profile based on age or gender.
During all Parkinson’s disease phase 2/3 clinical trials, the long-term safety profile was similar to that observed with shorter duration exposure.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AZILECT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Melanoma
5OVERDOSAGE
In a dose escalation study in patients on chronic levodopa therapy treated with 10 mg of AZILECT there were three reports of cardiovascular side effects (including hypertension and postural hypotension) which resolved following treatment discontinuation.
Although no cases of overdose have been observed with AZILECT during the clinical development program, the following description of presenting symptoms and clinical course is based upon overdose descriptions of nonselective MAO inhibitors.
The signs and symptoms of nonselective MAOI overdose may not appear immediately. Delays of up to 12 hours after ingestion of drug and the appearance of signs may occur. The peak intensity of the syndrome may not be reached until for a day following the overdose. Death has been reported following overdose; therefore, immediate hospitalization, with continuous patient observation and monitoring for at least two days following the ingestion of such drugs in overdose, is strongly recommended.
The severity of the clinical signs and symptoms of MAOI overdose varies and may be related to the amount of drug consumed. The central nervous and cardiovascular systems are prominently involved.
Signs and symptoms of MAOI overdose may include: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
There is no specific antidote for AZILECT overdose. The following suggestions are offered based upon the assumption that AZILECT overdose may be modeled after nonselective MAO inhibitor poisoning. Treatment of overdose with nonselective MAO inhibitors is symptomatic and supportive. Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required. Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential. For this reason, in cases of overdose with AZILECT, dietary tyramine restriction should be observed for several weeks to reduce the risk of hypertensive tyramine reaction.
A poison control center should be called for the most current treatment guidelines.
A postmarketing report described a single patient who developed a nonfatal serotonin syndrome after ingesting 100 mg of AZILECT in a suicide attempt. Another patient who was treated in error with 4 mg AZILECT daily and tramadol also developed a serotonin syndrome. One patient who was treated in error with 3 mg AZILECT daily experienced alternating episodes of vascular fluctuations consisting of hypertension and orthostatic hypotension.
6DESCRIPTION
AZILECT
Its structural formula is:
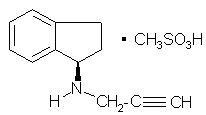
Rasagiline mesylate is a white to off-white powder, freely soluble in water or ethanol and sparingly soluble in isopropanol. Each AZILECT tablet for oral administration contains 0.5 mg or 1 mg of rasagiline (equivalent to 0.78 mg or 1.56 mg of rasagiline mesylate).
Each AZILECT tablet also contains the following inactive ingredients: mannitol, starch, pregelatinized starch, colloidal silicon dioxide, stearic acid, and talc.
7CLINICAL STUDIES
The effectiveness of AZILECT for the treatment of Parkinson’s disease was established in four 18- to 26-week, randomized, placebo-controlled trials, as initial monotherapy or adjunct therapy.
7.1Monotherapy Use of AZILECT
Study 1 was a double-blind, randomized, fixed-dose parallel group, 26-week study in early Parkinson’s disease patients not receiving any concomitant dopaminergic therapy at the start of the study. The majority of the patients were not treated with medications for Parkinson’s disease before receiving AZILECT.
In Study 1, 404 patients were randomly assigned to receive placebo (138 patients), AZILECT 1 mg/day (134 patients) or AZILECT 2 mg/day (132 patients). Patients were not allowed to take levodopa, dopamine agonists, selegiline, or amantadine, but could take stable doses of anticholinergic medication, if necessary. The average Parkinson’s disease duration was approximately 1 year (range 0 to 11 years).
The primary measure of effectiveness was the change from baseline in the total score of the Unified Parkinson’s Disease Rating Scale (UPDRS), [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)]. The UPDRS is a multi-item rating scale that measures the ability of a patient to perform mental and motor tasks as well as activities of daily living. A reduction in the score represents improvement and a beneficial change from baseline appears as a negative number.
AZILECT (1 or 2 mg once daily) was superior to placebo on the primary measure of effectiveness in patients receiving six months of treatment and not on dopaminergic therapy. The effectiveness of AZILECT 1 mg and 2 mg was comparable. Table 4 shows the results of Study 1. There were no differences in effectiveness based on age or gender between AZILECT 1 mg/day and placebo.
7.2Adjunct Use of AZILECT
Study 2 was a double-blind, randomized, placebo-controlled, parallel group, 18-week study, investigating AZILECT 1 mg as adjunct therapy to dopamine agonists without levodopa. Patients were on a stable dose of dopamine agonist (ropinirole, mean 8 mg/day or pramipexole, mean 1.5 mg/day) therapy for ≥ 30 days, but at doses not sufficient to control Parkinson’s disease symptoms.
In Study 2, 321 patients randomly received placebo (162 patients) or AZILECT 1 mg/day (159 patients) and had a post-baseline assessment. The average Parkinson’s disease duration was approximately 2 years (range 0.1 to 14.5 years).
The primary measure of effectiveness was the change from baseline in the total score of the Unified Parkinson’s Disease Rating Scale (UPDRS) [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)].
In Study 2, AZILECT 1 mg was superior to placebo on the primary measure of effectiveness (see Table 5).
*A negative change from baseline indicates improvement in the UPDRS
Secondary outcome assessment of the individual subscales of the UPDRS indicates that the UPDRS Part III motor subscale was primarily responsible for the overall AZILECT effect on the UPDRS score (see Table 6).
Study 3 and Study 4 were randomized, multinational trials conducted in more advanced Parkinson’s disease patients treated chronically with levodopa and experiencing motor fluctuations (including but not limited to, end of dose “wearing off,” sudden or random “off,” etc.). Study 3 was conducted in North America (U.S. and Canada) and compared AZILECT 0.5 mg and 1 mg daily to placebo. Study 4 was conducted outside of North America in Europe, Argentina, and Israel, and compared AZILECT 1 mg daily to placebo.
Patients had Parkinson’s disease for an average of 9 years (range 5 months to 33 years), had taken levodopa for an average of 8 years (range 5 months to 32 years), and had motor fluctuations for approximately 3 to 4 years (range 1 month to 23 years). Patients kept home Parkinson’s disease diaries just prior to baseline and at specified intervals during the trial. Diaries recorded one of the following four conditions for each half-hour interval over a 24-hour period: “ON” (period of relatively good function and mobility) as either “ON” with no dyskinesia or without troublesome dyskinesia, or “ON” with troublesome dyskinesia, “OFF” (period of relatively poor function and mobility) or asleep. “Troublesome” dyskinesia is defined as dyskinesia that interferes with the patient’s daily activity. All patients had inadequate control of their motor symptoms with motor fluctuations typical of advanced stage disease despite receiving levodopa/decarboxylase inhibitor. The average dose of levodopa taken with a decarboxylase inhibitor was approximately 700 to 800 mg (range 150 to 3000 mg/day). Patients continued their stable doses of additional anti-PD medications at entry into the trials. Approximately 65% of patients in both studies were also taking a dopamine agonist. In the North American study (Study 3), approximately 35% of patients took entacapone with levodopa/decarboxylase inhibitor. The majority of patients taking entacapone were also taking a dopamine agonist.
In Study 3 and Study 4, the primary measure of effectiveness was the change in the mean number of hours spent in the “OFF” state at baseline compared to the mean number of hours spent in the “OFF” state during the treatment period.
In Study 3, patients were randomly assigned to receive placebo (159 patients), AZILECT 0.5 mg/day (164 patients), or AZILECT 1 mg/day (149 patients) for 26 weeks. Patients averaged 6 hours daily in the “OFF” state at baseline as confirmed by home diaries.
In Study 4, patients were randomly assigned to receive placebo (229 patients), AZILECT 1 mg/day (231 patients) or a COMT inhibitor (active comparator), taken along with scheduled doses of levodopa/decarboxylase inhibitor (227 patients) for 18 weeks. Patients averaged 5.6 hours daily in the “OFF” state at baseline as confirmed by home diaries.
In Study 3 and Study 4, AZILECT 1 mg once daily reduced “OFF” time compared to placebo when added to levodopa in patients experiencing motor fluctuations (Tables 7 and 8). The lower dose (0.5 mg) of AZILECT also significantly reduced “OFF” time (Table 7), but had a numerically smaller effect than the 1 mg dose of AZILECT. In Study 4, the active comparator also reduced “OFF” time when compared to placebo.
In Study 3 and Study 4, dose reduction of levodopa was allowed within the first 6 weeks, if dopaminergic side effects developed including dyskinesia or hallucinations. In Study 3, the levodopa dose was reduced in 8% of patients in the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1 mg/day AZILECT groups, respectively. When levodopa was reduced, the dose was reduced by 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day groups, respectively. In Study 4, levodopa dose reduction occurred in 6% of patients in the placebo group and in 9% in the AZILECT 1 mg/day groups, respectively. When levodopa was reduced, it was reduced by 13% and 11% in the placebo and the AZILECT groups, respectively.
There were no differences in effectiveness based on age or gender between AZILECT 1 mg/day and placebo.
Several secondary outcome assessments in the two studies showed statistically significant improvements with rasagiline. These included effects on the activities of daily living (ADL) subscale of the UPDRS performed during an “OFF” period and the motor subscale of the UPDRS performed during an “ON” period. In both scales, a negative response represents improvement. Tables 9 and 10 show these results for Studies 3 and 4.
8HOW SUPPLIED/STORAGE AND HANDLING
AZILECT 0.5 mg Tablets:
White to off-white, round, flat, beveled tablets, debossed with “GIL 0.5” on one side and plain on the other side. Supplied as bottles of 30 tablets (NDC 68546-142-56).
AZILECT 1 mg Tablets:
White to off-white, round, flat, beveled tablets, debossed with “GIL 1” on one side and plain on the other side. Supplied as bottles of 30 tablets (NDC 68546-229-56).
Storage:
Store at 25°C (77°F) with excursions permitted to 15°-30°C (59°-86°F).
9PATIENT COUNSELING INFORMATION
Hypertension
Advise patients that treatment with recommended doses of AZILECT may be associated with elevations of blood pressure. Tell patients who experience elevation of blood pressure while taking AZILECT to contact their healthcare provider.
The risk of using higher than recommended daily doses of AZILECT should be explained, and a brief description of the tyramine associated hypertensive reaction provided.
Advise patients to avoid certain foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of AZILECT because of the potential for large increases in blood pressure. If patients eat foods very rich in tyramine and do not feel well soon after eating, they should contact their healthcare provider
Serotonin Syndrome
Tell patients to inform their physician if they are taking, or planning to take, any prescription or over-the-counter drugs, especially antidepressants and over-the-counter cold medications, since there is a potential for interaction with AZILECT. Because patients should not use meperidine or certain other analgesics with AZILECT, they should contact their healthcare provider before taking analgesics
Falling Asleep During Activities of Daily Living and Somnolence
Advise and alert patients about the potential for sedating effects associated with AZILECT and other dopaminergic medications, including somnolence and particularly to the possibility of falling asleep while engaged in activities of daily living. Because somnolence can be a frequent adverse reaction with potentially serious consequences, patients should neither drive a car nor engage in other potentially dangerous activities until they have gained sufficient experience with AZILECT and other dopaminergic medications to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Patients should not drive, operate machinery, or work at heights during treatment if they have previously experienced somnolence and/or have fallen asleep without warning prior to use of AZILECT.
Because of possible additive effects, advise patients to exercise caution when patients are taking other sedating medications, alcohol, or other central nervous system depressants (e.g., benzodiazepines, antipsychotics, antidepressants) in combination with AZILECT or when taking concomitant medications that increase plasma levels of rasagiline (e.g., ciprofloxacin)
Ciprofloxacin or Other CYP1A2 Inhibitors
Inform patients that they should contact their healthcare provider of AZILECT if they take ciprofloxacin or a similar drug that could increase blood levels of rasagiline because of the need to adjust the dose of AZILECT
Hepatic Impairment
Tell patients who have hepatic problems to contact their healthcare provider regarding possible changes in AZILECT dosing
Hypotension / Orthostatic Hypotension
Patients should be advised that they may develop orthostatic hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Hypotension and/or orthostatic symptoms may occur more frequently during initial therapy or with an increase in dose at any time (cases have been seen after weeks of treatment). Accordingly, patients should be cautioned against standing up rapidly after sitting or lying down, especially if they have been doing so for prolonged periods, and especially, at the initiation of treatment with AZILECT
Dyskinesia
Advise patients taking AZILECT as adjunct to levodopa that there is a possibility of dyskinesia or increased dyskinesia
Hallucinations / Psychotic-Like Behavior
Inform patients that hallucinations or other manifestations of psychotic-like behavior can occur when taking AZILECT. Advise patients that, if they have a major psychotic disorder, that AZILECT should not ordinarily be used because of the risk of exacerbating the psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of AZILECT
Impulse Control/Compulsive Behaviors
Advise patients that they may experience intense urges to gamble, increased sexual urges, other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease (including AZILECT). Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges, or other urges while being treated with AZILECT. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges, or other intense urges while taking AZILECT. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking AZILECT
Withdrawal-Emergent Hyperpyrexia and Confusion
Tell patients to contact their healthcare provider if they wish to discontinue AZILECT
Missing Dose
Instruct patients to take AZILECT as prescribed. If a dose is missed, the patient should not double-up the dose of AZILECT. The next dose should be taken at the usual time on the following day.
Pregnancy
Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant

AZI-004
Distributed by: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054
©2020 Teva Neuroscience, Inc.
10Package/Label Display Panel
NDC 68546-142-56

11Package/Label Display Panel
NDC 68546-229-56
