Brand Name
Zokinvy
Generic Name
Lonafarnib
View Brand Information FDA approval date: November 20, 2020
Classification: Farnesyltransferase Inhibitor
Form: Capsule
What is Zokinvy (Lonafarnib)?
ZOKINVY is indicated in patients 12 months of age and older with a body surface area of.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 2a, Randomized, Open-Label Study to Determine the Optimal Dose and Evaluate the Safety, Tolerability, and Pharmacokinetics of Progerinin in Patients with Hutchinson-Gilford Progeria Syndrome (HGPS)
Summary: Researchers will compare treatment with progerinin plus lonafarnib vs lonafarnib alone to assess optimal dosing, safety, tolerability, and pharmacokinetics in patients with Hutchinson-Gilford Progeria Syndrome (HGPS). Subjects in the randomized study arms will continue to take the standard of care (SOC), lonafarnib, and will be randomized to either take SOC alone or in combination with progerinin.
Related Latest Advances
Brand Information
Zokinvy (lonafarnib)
1INDICATIONS AND USAGE
ZOKINVY is indicated in patients 12 months of age and older with a body surface area (BSA) of 0.39 m
- To reduce the risk of mortality in Hutchinson-Gilford Progeria Syndrome (HGPS)
- For the treatment of processing-deficient Progeroid Laminopathies with either:
Limitations of Use
ZOKINVY is not indicated for other Progeroid Syndromes or processing-proficient Progeroid Laminopathies. Based upon its mechanism of action, ZOKINVY would not be expected to be effective in these populations.
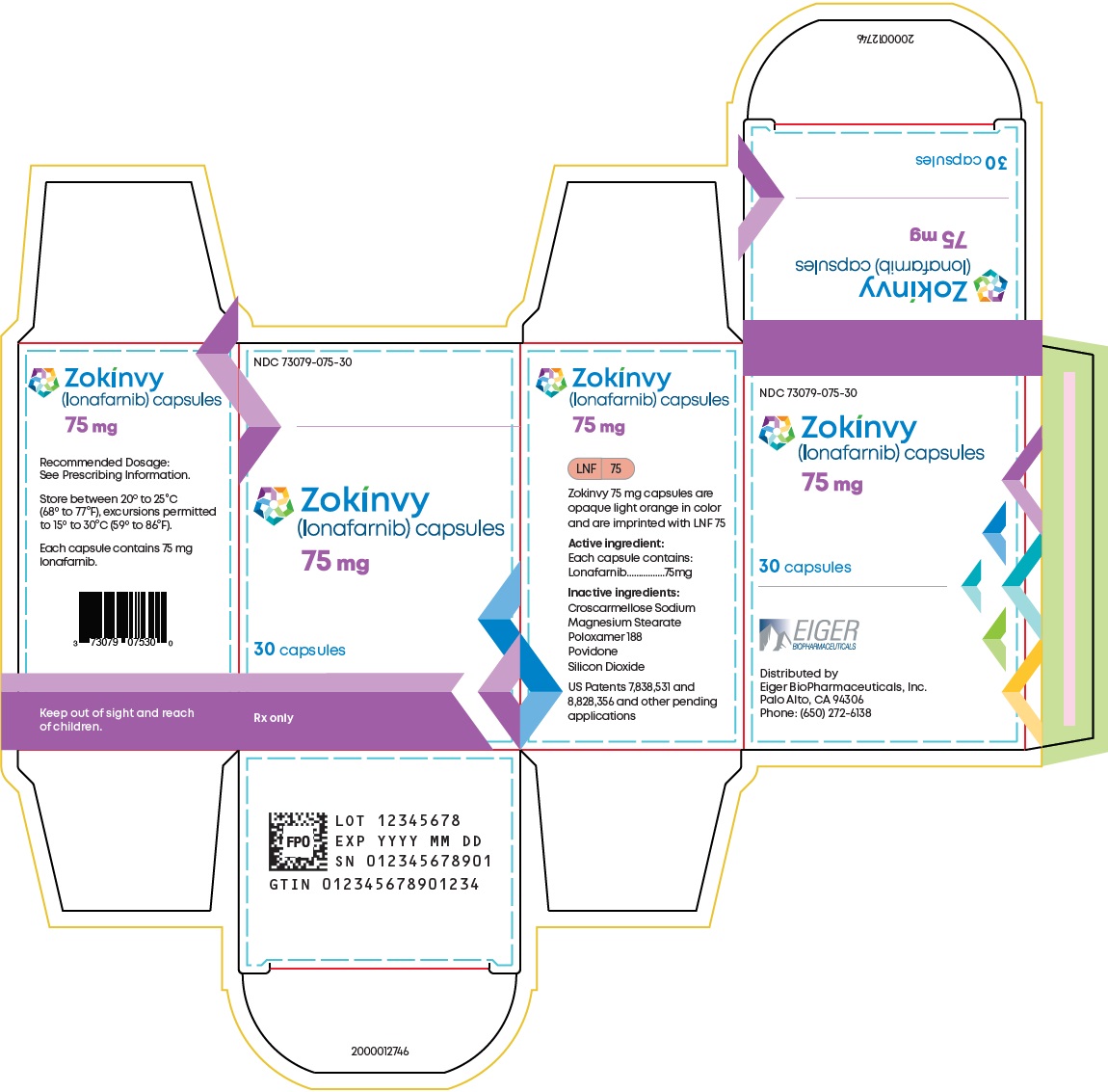
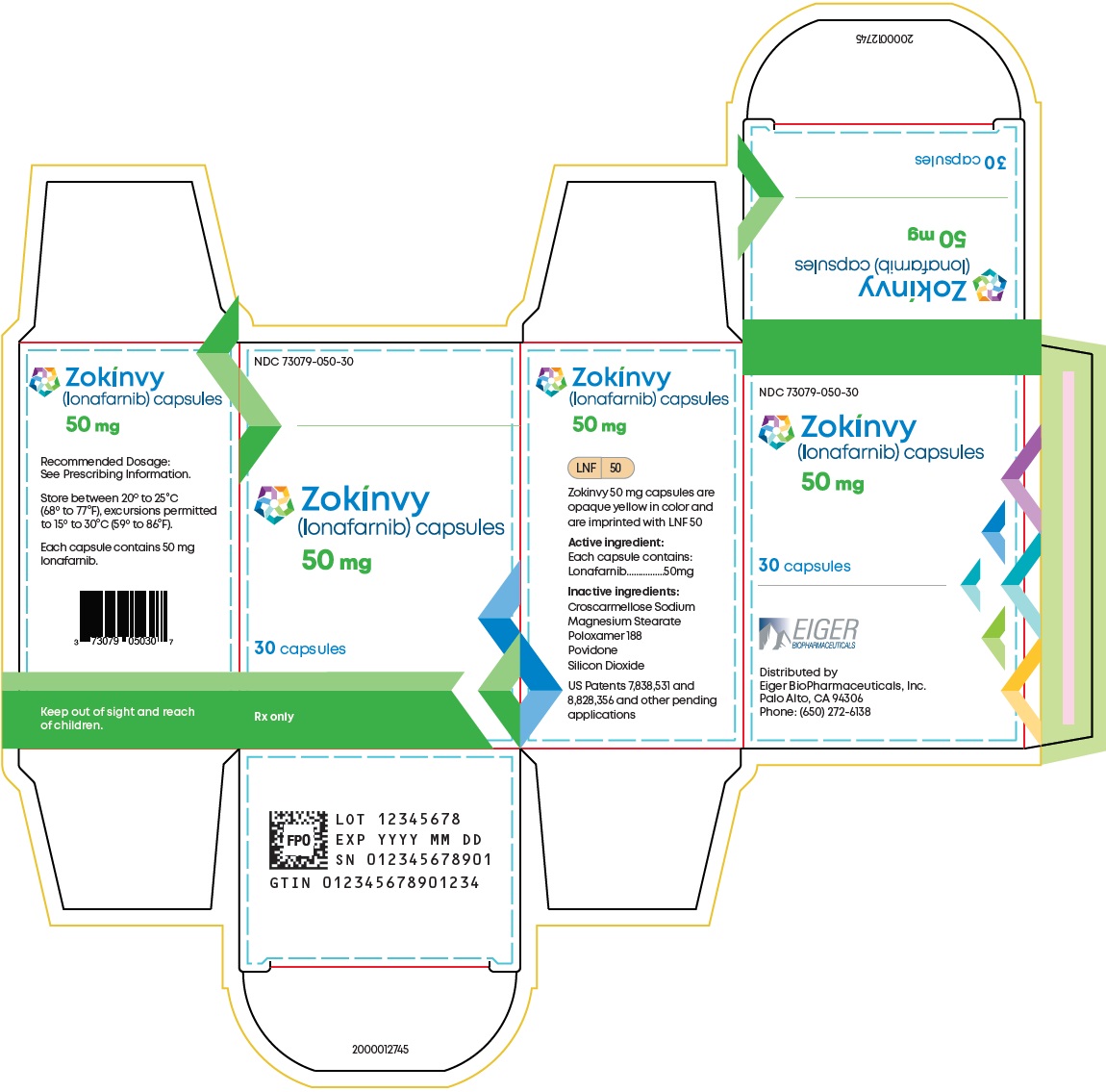
2DOSAGE FORMS AND STRENGTHS
Capsules:
- 50 mg, opaque yellow with “LNF” and “50” printed in black
- 75 mg, opaque light orange with “LNF” and “75” printed in black
3CONTRAINDICATIONS
ZOKINVY is contraindicated in patients taking:
- Strong CYP3A inhibitors
- Strong or moderate CYP3A inducers
- Midazolam
- Lovastatin, simvastatin, or atorvastatin
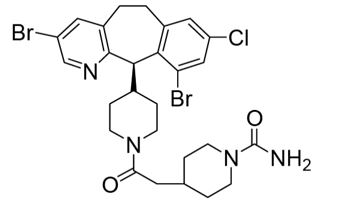
4DESCRIPTION
ZOKINVY (lonafarnib) is a farnesyltransferase inhibitor. The chemical name for lonafarnib is 4-[2-[4-[(11R)-3,10-dibromo-8-chloro-6,11-dihydro-5H- benzo[1,2] cyclohepta [2,4-b]pyridin-11-yl]piperidin-1-yl]-2- oxoethyl]piperidine-1-carboxamide. Its molecular formula is C
ZOKINVY (lonafarnib) capsules for oral administration contain 50 mg or 75 mg of lonafarnib as the active ingredient and the following inactive ingredients: croscarmellose sodium, magnesium stearate, poloxamer 188, povidone, and silicon dioxide. The capsule shells of both strengths contain gelatin, titanium dioxide, and yellow iron oxide; the 75 mg capsule also contains red iron oxide. The imprinting ink contains ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, and shellac.
5CLINICAL STUDIES
The efficacy of ZOKINVY is based on results from the Observational Cohort Survival Study, which retrospectively compared survival data from two Phase 2 studies in patients with HGPS to those from a natural history cohort.
Study 1 (NCT00425607) was a Phase 2 open-label, single-arm trial that evaluated the efficacy of ZOKINVY in 28 patients (26 with classic HGPS, one with non-classic HGPS, and one with Progeroid Laminopathy with
Following completion of Study 1, 26 patients enrolled in a second Phase 2 open label, single-arm trial (Study 2, NCT00916747) which consisted of two study phases. In the first phase of Study 2, patients received ZOKINVY with additional therapies for about 5 years. In the second phase of Study 2, patients received ZOKINVY 150 mg/m
There were 35 treatment naïve patients with HGPS enrolled into the second phase of Study 2. Among the 35 treated patients (22 males, 13 females), 34 (97.1%) patients had classic HGPS and 1 (2.9%) patient had non-classic HGPS. The median age was 6 years (range: 2 to 17 years). The body weight range was 6.7 to 22 kg and the BSA range was 0.42 to 0.90 m
Throughout Study 1 and Study 2, ZOKINVY was administered orally via capsules or the capsule contents were mixed with Ora Blend SF or Ora-Plus and administered orally as a suspension.
Table 8: Survival Analysis Summary for Patients with HGPS
Figure 1: Kaplan-Meier Survival Curves for Follow-up Time Censored at Last Follow-up for Patients with HGPS
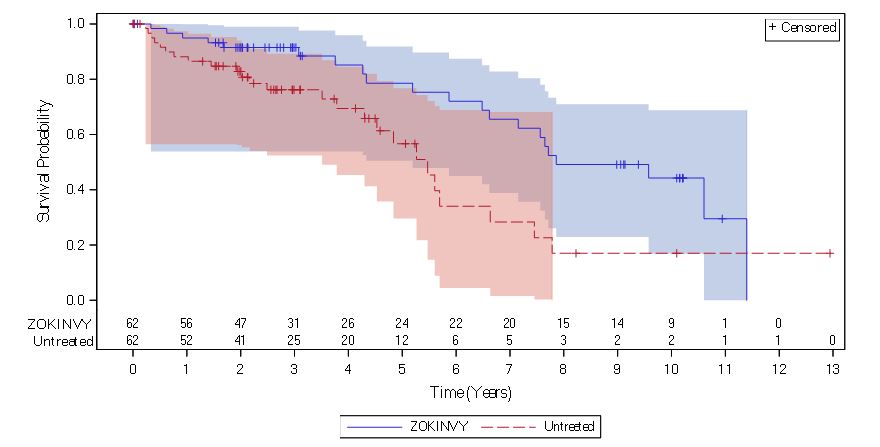
Note: The Kaplan-Meier (KM) survival curve for the ZOKINVY-treated patients is indicated with a solid line; the curve for the untreated patients is indicated with a dashed line. The shaded regions in blue and red represent the 95% confidence bands for the treated and untreated KM survival curves, respectively.
6HOW SUPPLIED/STORAGE AND HANDLING
ZOKINVY is supplied as:
- 50 mg capsules: Size 4 hard capsule, opaque yellow with “LNF” and “50” printed in black.
- 75 mg capsules: Size 3 hard capsule, opaque light orange with “LNF and “75” printed in black.
Store at 20°C-25°C (68°F-77°F), excursions permitted to 15°C-30ºC (59°F-86°F) [see USP Controlled Room Temperature].
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Dosing [see Dosage and Administration (
- Advise patients and caregivers that ZOKINVY should be taken twice daily with the morning and evening meals.
- Inform patients and caregivers that if a dose is missed, the next dose should be given as soon as possible up to 8 hours prior to the next scheduled dose. If less than 8 hours remain before the next scheduled dose, the patient should skip the missed dose and resume taking ZOKINVY at the next scheduled dose.
Preparation and Administration [see Dosage and Administration (
- Advise patients to swallow the capsule whole with water. The capsules should not be chewed.
- For patients unable to swallow capsules, advise patients and caregivers that the contents of ZOKINVY can be mixed with Ora Blend SF or Ora-Plus. For patients unable to access or tolerate Ora Blend SF or Ora-Plus, the contents of ZOKINVY can be mixed with orange juice or applesauce. Advise patients not to mix the contents of ZOKINVY with juice containing grapefruit or Seville oranges. Advise patients and caregivers that the mixture must be prepared fresh for each dose and taken within approximately 10 minutes of mixing.
- Advise patients and caregivers to read and carefully follow the instructions for administering the capsule contents in Ora Blend SF, Ora-Plus, orange juice or applesauce
QTc Interval Prolongation [see Warnings and Precautions (
- Inform patients and caregivers that ZOKINVY causes QTc interval prolongation and may increase the risk of Torsades de pointes, other ventricular arrhythmias, and sudden death.
- Instruct patients or caregivers to notify their healthcare provider if they experience symptoms such as dizziness, lightheadedness, heart palpitations, or loss of consciousness.
- Instruct patients to inform their healthcare provider if they are taking any other medications that may prolong the QTc interval.
Drug Interactions [see Dosage and Administration (
Inform patients and caregivers that ZOKINVY may interact with several drugs. Advise patients and their caregivers to inform their healthcare provider before starting or discontinuing a prescription or non-prescription drug, supplement, or strong CYP3A inhibitor.
Inform patients and caregivers that ZOKINVY may interact with several drugs. Advise patients and their caregivers to inform their healthcare provider before starting or discontinuing a prescription or non-prescription drug, supplement, or strong CYP3A inhibitor.
Nephrotoxicity [see Warnings and Precautions (
Inform the patient and caregiver of the risk of kidney damage.
Inform the patient and caregiver of the risk of kidney damage.
Retinal Toxicity [see Warnings and Precautions (
Inform the patient and caregiver of the risk of developing difficulty with night vision. Advise patients and caregivers to contact their healthcare provider if they experience a change in vision.
Inform the patient and caregiver of the risk of developing difficulty with night vision. Advise patients and caregivers to contact their healthcare provider if they experience a change in vision.
Gastrointestinal Adverse Reactions [see Dosage and Administration (
Inform patients and caregivers that gastrointestinal adverse reactions are common with ZOKINVY. These include, but are not limited to, vomiting, diarrhea, and nausea. Advise patients and caregivers to contact their healthcare provider if these adverse reactions persist.
Inform patients and caregivers that gastrointestinal adverse reactions are common with ZOKINVY. These include, but are not limited to, vomiting, diarrhea, and nausea. Advise patients and caregivers to contact their healthcare provider if these adverse reactions persist.
Hypertension [see Adverse Reactions (
Inform patients and caregivers that blood pressure may increase while taking ZOKINVY. Symptoms of hypertension may include headaches, shortness of breath, nosebleeds, flushing, dizziness, or chest pain. Advise patients and caregivers to contact their healthcare provider if these adverse reactions occur.
Inform patients and caregivers that blood pressure may increase while taking ZOKINVY. Symptoms of hypertension may include headaches, shortness of breath, nosebleeds, flushing, dizziness, or chest pain. Advise patients and caregivers to contact their healthcare provider if these adverse reactions occur.
Impaired Fertility [see Warnings and Precautions (
Inform females and males of reproductive potential that ZOKINVY may impact pubertal development and impair fertility.
Inform females and males of reproductive potential that ZOKINVY may impact pubertal development and impair fertility.
Embryo-Fetal Toxicity [see Warnings and Precautions (
Inform pregnant women and female patients of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ZOKINVY.
Inform pregnant women and female patients of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ZOKINVY.
Manufactured for:
8PRINCIPAL DISPLAY PANEL - NDC: 73079-050-30 - 50 mg 30-count Bottle Label

9PRINCIPAL DISPLAY PANEL - NDC: 73079-050-30 - 50 mg 30-count Bottle Carton Label
10PRINCIPAL DISPLAY PANEL - NDC: 73079-075-30 - 75 mg 30-count Bottle Label

11PRINCIPAL DISPLAY PANEL - NDC: 73079-075-30 - 75 mg 30-count Bottle Carton Label