Generic Name
Azithromycin
Brand Names
Zithromax, Azasite
FDA approval date: September 28, 1994
Classification: Macrolide Antimicrobial
Form: Injection, Tablet, Powder, Suspension, Solution
What is Zithromax (Azithromycin)?
Azithromycin for oral suspension USP is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. Recommended dosages and durations of therapy in adult and pediatric patient populations vary in these indications.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Zithromax (azithromycin dihydrate)
1INDICATIONS AND USAGE
ZITHROMAX (azithromycin) is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. Recommended dosages and durations of therapy in adult and pediatric patient populations vary in these indications.
1.1Adult Patients
- Acute bacterial exacerbations of chronic bronchitis due to
- Acute bacterial sinusitis due to
- Community-acquired pneumonia due to
- Pharyngitis/tonsillitis caused by
- Uncomplicated skin and skin structure infections due to
- Urethritis and cervicitis due to
- Genital ulcer disease in men due to
1.2Pediatric Patients
[see
- Acute otitis media
- Community-acquired pneumonia
- Pharyngitis/tonsillitis
1.3Limitations of Use
Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following:
- patients with cystic fibrosis,
- patients with nosocomial infections,
- patients with known or suspected bacteremia,
- patients requiring hospitalization,
- elderly or debilitated patients, or
- patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
1.4Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ZITHROMAX (azithromycin) and other antibacterial drugs, ZITHROMAX (azithromycin) should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2DOSAGE FORMS AND STRENGTHS
ZITHROMAX 250 mg tablets are supplied as pink modified capsular shaped, engraved, film-coated tablets containing azithromycin dihydrate equivalent to 250 mg of azithromycin. ZITHROMAX 250 mg tablets are engraved with "PFIZER" on one side and "306" on the other, or "Pfizer" on one side and "ZTM 250" on the other. These are packaged in bottles and blister cards of 6 tablets (Z-PAKS
ZITHROMAX 500 mg tablets are supplied as pink modified capsular shaped, engraved, film-coated tablets containing azithromycin dihydrate equivalent to 500 mg of azithromycin. ZITHROMAX 500 mg tablets are engraved with "Pfizer" on one side and "ZTM500" on the other. These are packaged in bottles and blister cards of 3 tablets (TRI-PAKS™).
ZITHROMAX for oral suspension after constitution contains a flavored suspension. ZITHROMAX for oral suspension is supplied to provide 100 mg/5 mL or 200 mg/5 mL suspension in bottles.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hypersensitivity
- Hepatotoxicity
- Infantile Hypertrophic Pyloric Stenosis (IHPS)
- QT Prolongation
- Cardiovascular Death
- Clostridioides difficile-Associated Diarrhea (CDAD) [see
- Exacerbation of Myasthenia Gravis
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, most of the reported side effects were mild to moderate in severity and were reversible upon discontinuation of the drug. Potentially serious adverse reactions of angioedema and cholestatic jaundice were reported. Approximately 0.7% of the patients (adults and pediatric patients) from the 5-day multiple-dose clinical trials discontinued ZITHROMAX (azithromycin) therapy because of treatment-related adverse reactions. In adults given 500 mg/day for 3 days, the discontinuation rate due to treatment-related adverse reactions was 0.6%. In clinical trials in pediatric patients given 30 mg/kg, either as a single dose or over 3 days, discontinuation from the trials due to treatment-related adverse reactions was approximately 1%. Most of the adverse reactions leading to discontinuation were related to the gastrointestinal tract, e.g., nausea, vomiting, diarrhea, or abdominal pain.
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of azithromycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported with azithromycin during the postmarketing period in adult and/or pediatric patients for which a causal relationship may not be established include:
Allergic:Arthralgia, edema, urticaria, and angioedema.
Cardiovascular:Arrhythmias including ventricular tachycardia and hypotension. There have been reports of QT prolongation, torsades de pointes, and cardiovascular death.
Gastrointestinal:Anorexia, constipation, dyspepsia, flatulence, vomiting/diarrhea, pseudomembranous colitis, pancreatitis, oral candidiasis, pyloric stenosis, and reports of tongue discoloration.
General:Asthenia, paresthesia, fatigue, malaise, and anaphylaxis.
Genitourinary:Interstitial nephritis and acute renal failure and vaginitis.
Hematopoietic:Thrombocytopenia.
Liver/Biliary:Abnormal liver function, hepatitis, cholestatic jaundice, hepatic necrosis, and hepatic failure. [see
Nervous System:Convulsions, dizziness/vertigo, headache, somnolence, hyperactivity, nervousness, agitation, and syncope.
Psychiatric:Aggressive reaction and anxiety.
Skin/Appendages:Pruritus serious skin reactions including erythema multiforme, AGEP, Stevens-Johnson Syndrome, toxic epidermal necrolysis, and DRESS.
Special Senses:Hearing disturbances including hearing loss, deafness and/or tinnitus, and reports of taste/smell perversion and/or loss.
4OVERDOSAGE
Adverse reactions experienced at higher than recommended doses were similar to those seen at normal doses particularly nausea, diarrhea, and vomiting. In the event of overdosage, general symptomatic and supportive measures are indicated as required.
5DESCRIPTION
ZITHROMAX (azithromycin tablets and azithromycin for oral suspension) contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl) oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.00. Azithromycin has the following structural formula:
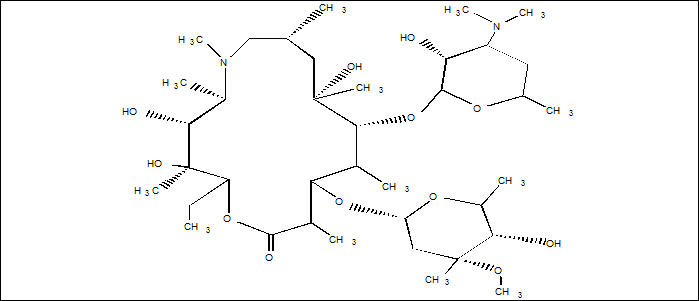
Azithromycin, as the dihydrate, is a white crystalline powder with a molecular formula of C38H72N2O12∙2H2O and a molecular weight of 785.0.
ZITHROMAX is supplied as tablets containing azithromycin dihydrate equivalent to either 250 mg or 500 mg azithromycin and the following inactive ingredients: dibasic calcium phosphate anhydrous, pregelatinized starch, sodium croscarmellose, magnesium stearate, sodium lauryl sulfate, hypromellose, lactose, titanium dioxide, triacetin, and D&C Red #30 aluminum lake.
ZITHROMAX for oral suspension is supplied in bottles containing azithromycin dihydrate powder equivalent to 300 mg, 600 mg, 900 mg, or 1200 mg azithromycin per bottle and the following inactive ingredients: sucrose; sodium phosphate, tribasic, anhydrous; hydroxypropyl cellulose; xanthan gum; FD&C Red #40; and spray dried artificial cherry, creme de vanilla, and banana flavors. After constitution, each 5 mL of suspension contains 100 mg or 200 mg of azithromycin.
6HOW SUPPLIED/STORAGE AND HANDLING
ZITHROMAX is supplied in the following strengths and package configurations:
ZITHROMAX tablets should be stored between 15° to 30°C (59° to 86°F).
ZITHROMAX for oral suspension after constitution contains a flavored suspension. ZITHROMAX for oral suspension is supplied to provide 100 mg/5 mL or 200 mg/5 mL suspension in bottles as follows:
[see for constitution instructions with each bottle type.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
8Patient Information
ZITHROMAX
(azithromycin)
Tablets
(azithromycin)
Tablets
ZITHROMAX
(azithromycin)
Oral Suspension
(azithromycin)
Oral Suspension
Read this Patient Information leaflet before you start taking ZITHROMAX and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is ZITHROMAX?
ZITHROMAX is a macrolide antibiotic prescription medicine used in adults 18 years or older to treat certain infections caused by certain germs called bacteria. These bacterial infections include:
- acute worsening of chronic bronchitis
- acute sinus infection
- community-acquired pneumonia
- infected throat or tonsils
- skin infections
- infections of the urethra or cervix
- genital ulcers in men
ZITHROMAX is also used in children to treat:
- ear infections
- community-acquired pneumonia
- infected throat or tonsils
Azithromycin should not be taken by people who cannot tolerate oral medications because they are very ill or have certain other risk factors including:
- have cystic fibrosis
- have hospital acquired infections
- have known or suspected bacteria in the blood
- need to be in the hospital
- are elderly
- have any medical problems that can lower the ability of the immune system to fight infections
ZITHROMAX is not for viral infections such as the common cold.
It is not known if ZITHROMAX is safe and effective for genital ulcers in women.
It is not known if ZITHROMAX is safe and effective for children with ear infections, sinus infections, and community-acquired pneumonia under 6 months of age.
It is not known if ZITHROMAX is safe and effective for infected throat or tonsils in children under 2 years of age.
Who should not take ZITHROMAX?
Do not take ZITHROMAX if you:
- have had a severe allergic reaction to certain antibiotics known as macrolides or ketolides including azithromycin and erythromycin.
- have a history of cholestatic jaundice or hepatic dysfunction that happened with the use of azithromycin.
What should I tell my healthcare provider before taking ZITHROMAX?
Before you take ZITHROMAX, tell your healthcare provider if you:
- have pneumonia
- have cystic fibrosis
- have known or suspected bacteremia (bacterial infection in the blood)
- have liver or kidney problems
- have an irregular heartbeat, especially a problem called "QT prolongation"
- have a problem that causes muscle weakness (myasthenia gravis)
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if ZITHROMAX will harm your unborn baby.
- are breastfeeding or plan to breastfeed. ZITHROMAX has been reported to pass into breast milk. Talk to your healthcare provider about the best way to feed your baby while you take ZITHROMAX.
Contact your healthcare provider immediately if you are giving ZITHROMAX to a young child (less than 6 weeks of age) and he or she vomits or becomes irritable when fed.
Tell your healthcare provider about all the medicines you take,including prescription and non-prescription medicines, vitamins, and herbal supplements.
ZITHROMAX and other medicines may affect each other causing side effects. ZITHROMAX may affect the way other medicines work, and other medicines may affect how ZITHROMAX works.
Especially tell your healthcare provider if you take:
- nelfinavir
- a blood thinner (warfarin)
- digoxin
- colchicine
- phenytoin
- an antacid that contains aluminum or magnesium
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take ZITHROMAX?
- Take ZITHROMAX exactly as your healthcare provider tells you to take it.
- ZITHROMAX can be taken with or without food.
- If you take ZITHROMAX Oral Suspension, shake the bottle well just before you take it.
- Do not skip any doses of ZITHROMAX or stop taking it, even if you begin to feel better, until you finish your prescribed treatment unless you have a serious allergic reaction or your healthcare provider tells you to stop taking ZITHROMAX.
- If the bacteria becomes resistant to ZITHROMAX, ZITHROMAX and other antibiotic medicines may not work for you in the future.
- If you take too much ZITHROMAX, call your healthcare provider or get medical help right away.
What are the possible side effects of ZITHROMAX?
ZITHROMAX can cause serious side effects, including:
- Serious allergic reactions.Allergic reactions can happen in people taking azithromcyin the active ingredient in ZITHROMAX, even after only 1 dose. Stop taking ZITHROMAX and get emergency medical help right away if you have any of the following symptoms of a severe allergic reaction:
- trouble breathing or swallowing
- swelling of the lips, tongue, face
- throat tightness, hoarseness
- rapid heartbeat
- faintness
- skin rash (hives)
- new onset of fever and swollen lymph nodes
- Stop taking ZITHROMAX at the first sign of a skin rash and call your healthcare provider. Skin rash may be a sign of a more serious reaction to ZITHROMAX.
- Liver damage (hepatotoxicity).Hepatotoxicity can happen in people who take ZITHROMAX. Call your healthcare provider right away if you have unexplained symptoms such as:
- Stop taking ZITHROMAX and tell your healthcare provider right away if you have yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to ZITHROMAX (a liver problem).
- Serious heart rhythm changes that can be life-threatening, including heart stopping (cardiac arrest), QT prolongation, torsades de pointes, feeling that your heart is pounding or racing (palpitations), chest discomfort, or irregular heartbeat.
Tell your healthcare provider right away if you or your child feel a fast or irregular heartbeat, get dizzy or faint. ZITHROMAX may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:- who are elderly
- with a family history of prolonged QT interval
- with low blood potassium
- who take certain medicines to control heart rhythm (antiarrhythmics)
- Worsening of myasthenia gravis (a problem that causes muscle weakness).Certain antibiotics like ZITHROMAX may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Call your healthcare provider right away if you have any worsening muscle weakness or breathing problems.
- Diarrhea.Tell your healthcare provider right away if you have watery diarrhea, diarrhea that does not go away, or bloody stools. You may experience cramping and a fever. This could happen after you have finished your ZITHROMAX.
The most common side effects of ZITHROMAX include:- nausea
- stomach pain
- vomiting
These are not all the possible side effects of ZITHROMAX. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZITHROMAX?
- Store ZITHROMAX Tablets at 59°F to 86°F (15°C to 30°C).
- Store ZITHROMAX Oral Suspension at 41°F to 86°F (5°C to 30°C).
- Keep ZITHROMAX Oral Suspension in a tightly closed container.
- Safely throw away any medicine that is out of date or no longer needed.
Keep ZITHROMAX and all medicines out of the reach of children.
General information about the safe and effective use of ZITHROMAX.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use ZITHROMAX for a condition for which it was not prescribed.
Do not give ZITHROMAX to other people, even if they have the same symptoms you have.
It may harm them.
This Patient Information leaflet summarizes the most important information about ZITHROMAX. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about ZITHROMAX that is written for health professionals.
For more information, go to www.zithromax.com or call 1-800-438-1986.
What are the ingredients in ZITHROMAX Tablets and Oral Suspension?
ZITHROMAX Tablets and Oral Suspension
Active ingredient: azithromycin dehydrate
ZITHROMAX Tablets:
Inactive ingredients: dibasic calcium phosphate anhydrous, pregelatinized starch, sodium croscarmellose, magnesium stearate, sodium lauryl sulfate, hypromellose, lactose, titanium dioxide, triacetin, and D&C Red #30 aluminum lake.
ZITHROMAX Oral Suspension:
Inactive ingredients: sucrose; sodium phosphate, tribasic, anhydrous; hydroxypropyl cellulose; xanthan gum; FD&C Red #40; and spray dried artificial cherry, creme de vanilla, and banana flavors.
This Patient Information has been approved by the U.S. Food and Drug Administration.

LAB-0372-7.0
9PRINCIPAL DISPLAY PANEL - 250 mg - 30 Tablet Bottle Label
NDC 0069-3060-30
Pfizer
Zithromax®
(azithromycin) tablets
(azithromycin) tablets
250 mg*
30 Tablets

10PRINCIPAL DISPLAY PANEL - 250 mg - 6 ct. Blister Pack
Zithromax®
(AZITHROMYCIN)
250 mg*
Tablets
(AZITHROMYCIN)
250 mg*
Tablets
Costs less
Z
Z-PAK ®
Z-PAK ®
A full course
Rx only
6 TABLETS

11PRINCIPAL DISPLAY PANEL - 250 mg - 10 ct. Blister Pack
Zithromax®
(azithromycin) Tablet
(azithromycin) Tablet
250 mg
Distributed by
13074600
EXP & LOT AREA

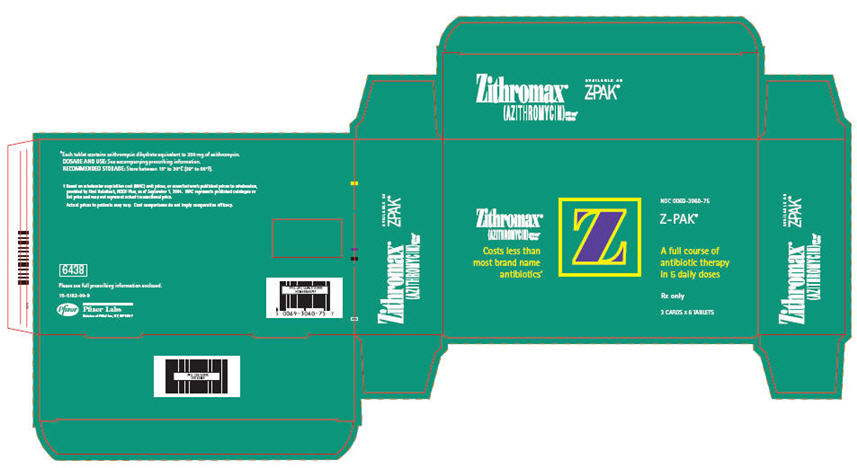
12PRINCIPAL DISPLAY PANEL - 250 mg - 18 Tablet Blister Pack
Zithromax
(AZITHROMYCIN) 250 mg*
Tablets
(AZITHROMYCIN) 250 mg*
Tablets
Costs less than
NDC 0069-3060-75
Z-PAK
A full course of
Rx only
3 CARDS x 6 TABLETS
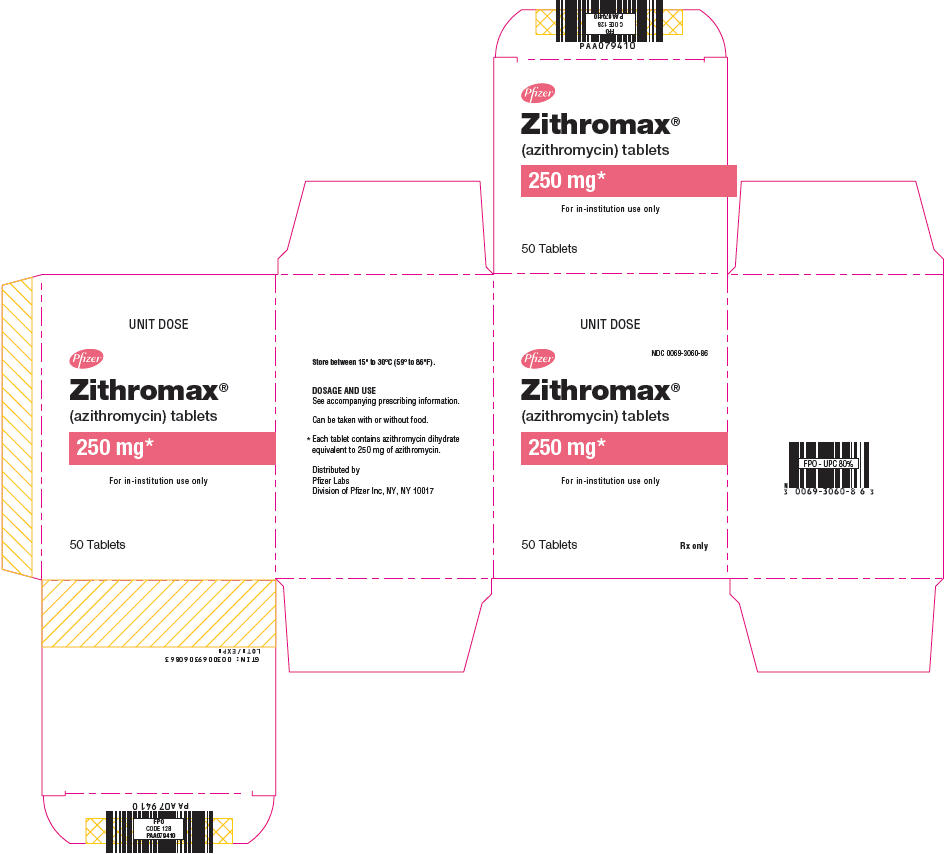
13PRINCIPAL DISPLAY PANEL - 250 mg Tablet Blister Pack Box
UNIT DOSE
NDC 0069-3060-86
Pfizer
Zithromax®
(azithromycin) tablets
(azithromycin) tablets
250 mg*
For in-institution use only
50 Tablets
14PRINCIPAL DISPLAY PANEL - 500 mg - 30 Tablet Bottle Label
NDC 0069-3070-30
Pfizer
Zithromax
500 mg*
30 Tablets
Rx only

15PRINCIPAL DISPLAY PANEL - 500 mg - 3 ct. Tablet Blister Pack
Rx only
Z
Zithromax®
(AZITHROMYCIN)500 mg Tablets
(AZITHROMYCIN)500 mg Tablets
TRI-PAK™
3 tablets x 500 mg*
A full course of
antibiotic therapy in
3 DAILY DOSES
antibiotic therapy in
3 DAILY DOSES

16PRINCIPAL DISPLAY PANEL - 500 mg - 3 ct. Tablet Blister Carton
NDC 0069-3070-75
Z
Zithromax®
(AZITHROMYCIN)500 mg Tablets
(AZITHROMYCIN)500 mg Tablets
TRI-PAK™
3 Cards x Three 500 mg* Tablets

17PRINCIPAL DISPLAY PANEL - 300 mg Bottle Label
NDC 0069-3110-19
300 mg (15 mL when mixed)
Pfizer
Zithromax
100 mg* per 5 mL
CHERRY FLAVORED
Rx only

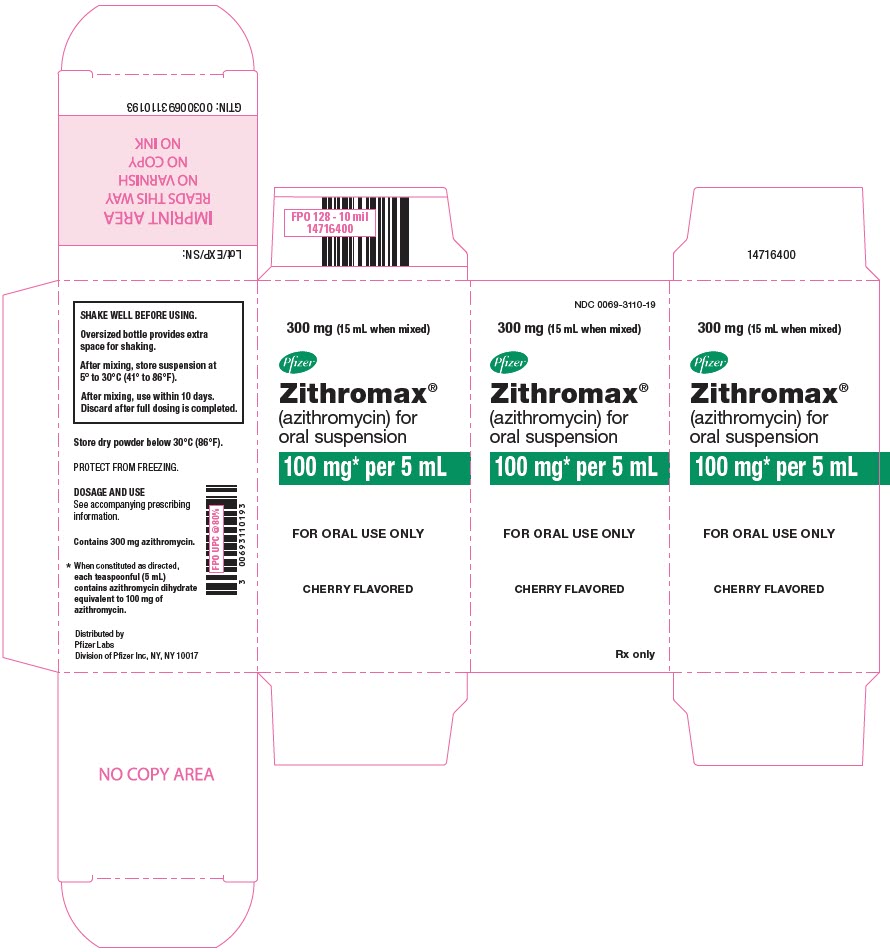
18PRINCIPAL DISPLAY PANEL - 300 mg Bottle Carton
NDC 0069-3110-19
300 mg (15 mL when mixed)
Pfizer
Zithromax
100 mg* per 5 mL
FOR ORAL USE ONLY
CHERRY FLAVORED
Rx only
19PRINCIPAL DISPLAY PANEL - 600 mg Bottle Label
NDC 0069-3120-19
600 mg (15 mL when mixed)
Pfizer
Zithromax
200 mg* per 5 mL
CHERRY FLAVORED
Rx only

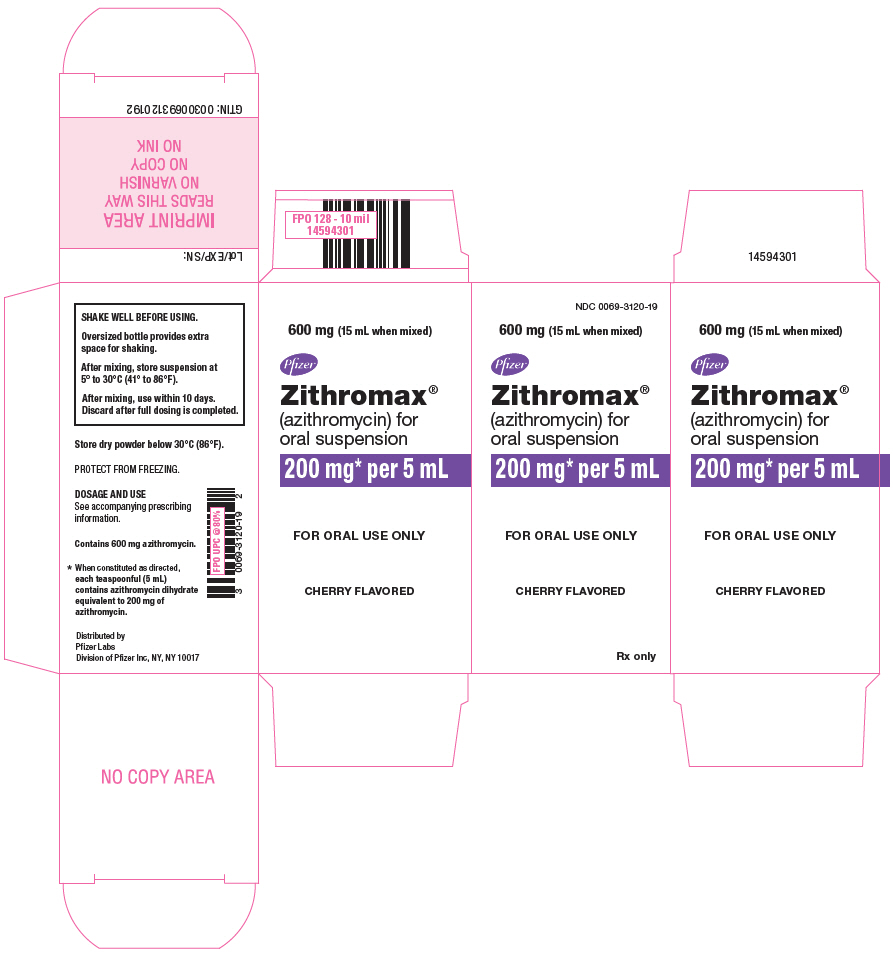
20PRINCIPAL DISPLAY PANEL - 600 mg Bottle Carton
NDC 0069-3120-19
600 mg (15 mL when mixed)
www.zithromax.com
Pfizer
Zithromax
200 mg* per 5 mL
FOR ORAL USE ONLY
CHERRY FLAVORED
Rx only
21PRINCIPAL DISPLAY PANEL - 900 mg Bottle Label
NDC 0069-3130-19
900 mg (22.5 mL when mixed)
Pfizer
Zithromax
200 mg* per 5 mL
CHERRY FLAVORED
Rx only

22PRINCIPAL DISPLAY PANEL - 900 mg Bottle Carton
NDC 0069-3130-19
900 mg (22.5 mL when mixed)
Pfizer
Zithromax
200 mg* per 5 mL
FOR ORAL USE ONLY
CHERRY FLAVORED
Rx only

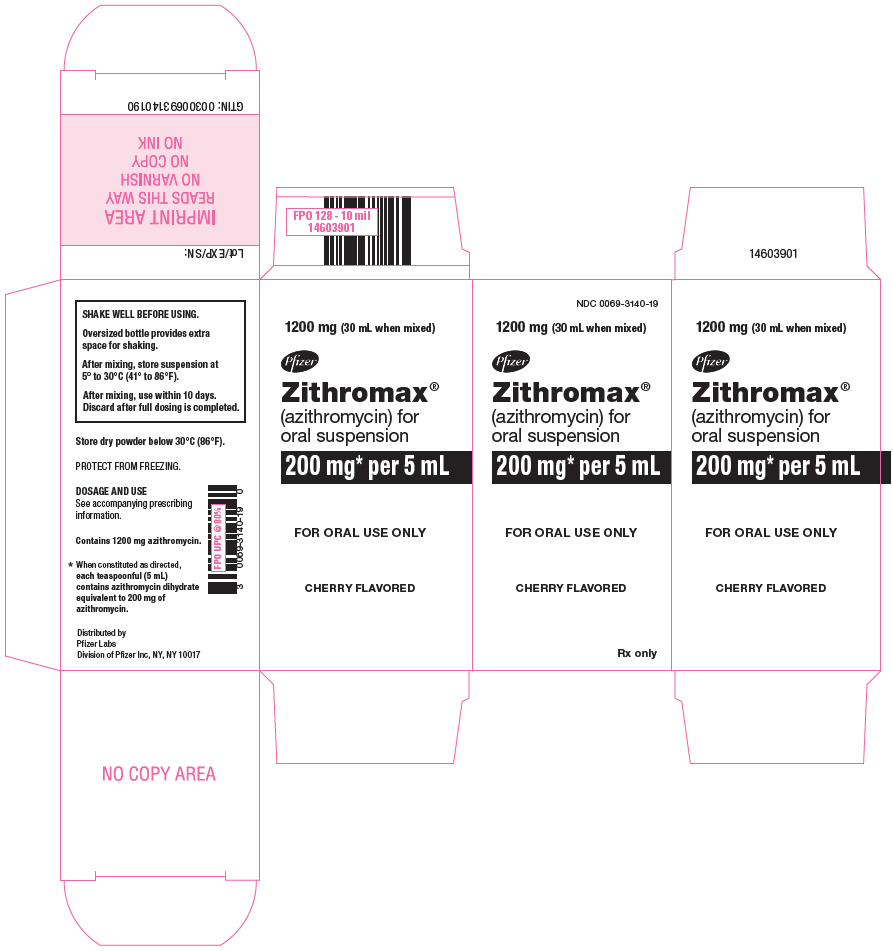
23PRINCIPAL DISPLAY PANEL - 1200 mg Bottle Label
NDC 0069-3140-19
1200 mg (30 mL when mixed)
Pfizer
Zithromax
200 mg* per 5 mL
CHERRY FLAVORED
Rx only

24PRINCIPAL DISPLAY PANEL - 1200 mg Bottle Carton
NDC 0069-3140-19
1200 mg (30 mL when mixed)
Pfizer
Zithromax
200 mg* per 5 mL
FOR ORAL USE ONLY
CHERRY FLAVORED
Rx only