Loteprednol Etabonate
What is Alrex (Loteprednol Etabonate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The Beads vs Vac trial is a multi-centre randomized controlled trial of 312 participants with a severe open tibia fracture requiring multiple irrigation and debridement surgeries. Eligible participants will be randomized to receive either an antibiotic bead pouch or negative pressure wound therapy (NPWT) for their temporary open fracture wound management. Outcomes will be assessed at 6 weeks, 3 mo...
Summary: Total hip replacement is the most successful treatment modern healthcare can offer patients to regain quality of life. Periprosthetic joint infection (PJI) is the most common and devastating complication after total hip replacement (THR). Between 0.5 to 2% of primary THR (first time hip replacement), and 8-10% of revision THR (replacement of a hip prosthesis) will become infected.1 The introductio...
Summary: A double-blind, active-controlled, multiple-ascending dose, safety study of aerosolized RSP-1502 in subjects with cystic fibrosis Pseudomonas aeruginosa lung infection.
Related Latest Advances
Brand Information

- NDC 24208-353-05 5 mL in a 7.5 mL bottle
- NDC 24208-353-10 10 mL in a 10 mL bottle
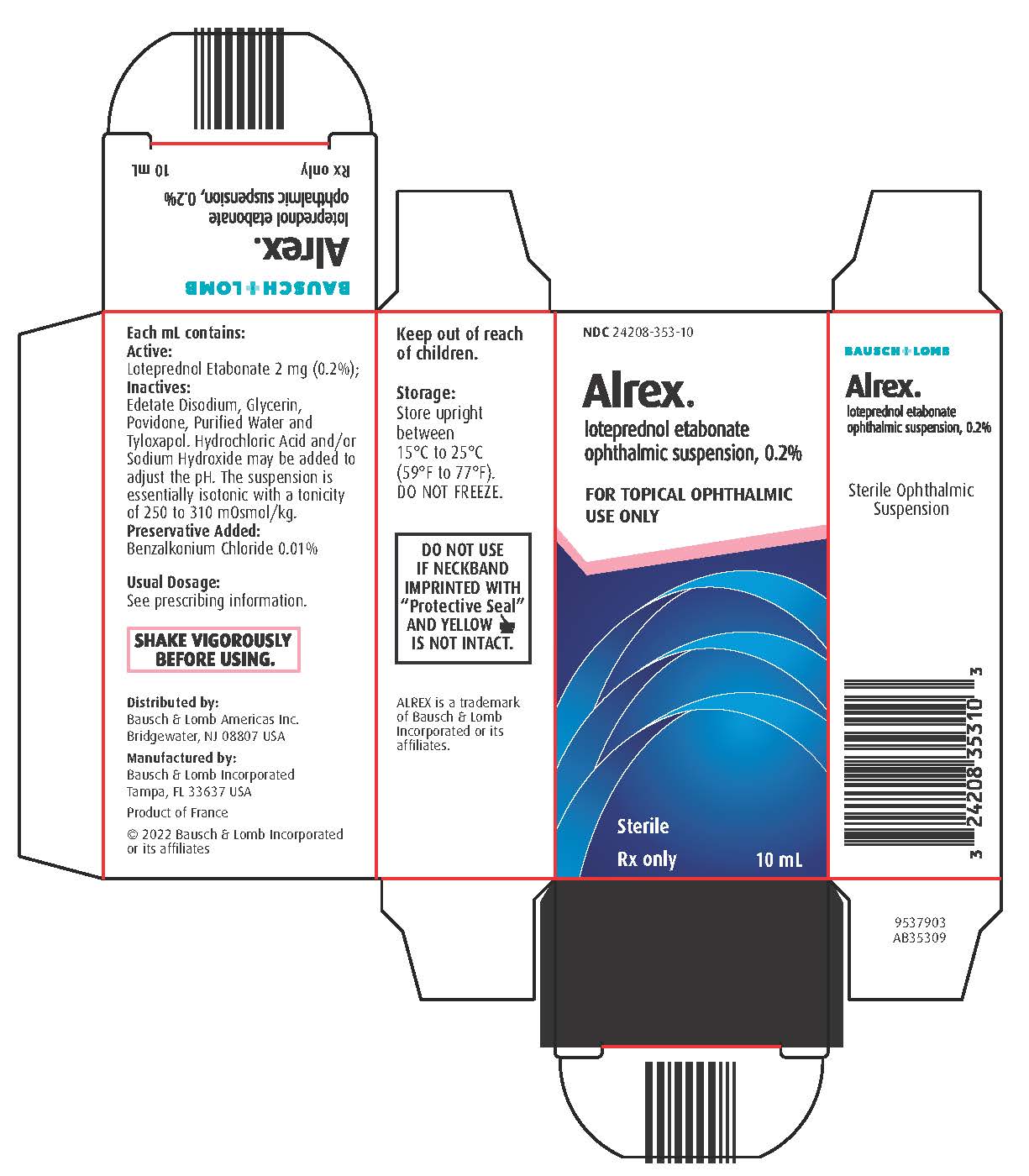
loteprednol etabonate
ophthalmic suspension, 0.2%
FOR OPHTHALMIC USE ONLY
Sterile
10 mL
