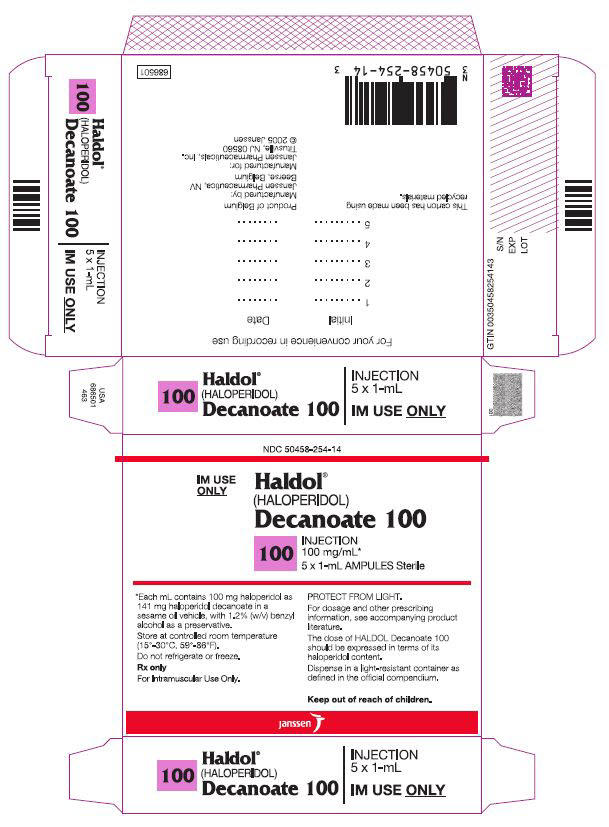
Haldol Decanoate
What is Haldol Decanoate (Decanoate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Medicines that reduce the amount of testosterone in the body are commonly used to treat prostate cancer. PRL-02 depot is a potential treatment for men with advanced prostate cancer. It is given by an injection into the muscle. Men with advanced prostate cancer can take part in this study. Their cancer has come back after previous cancer treatment, or the previous cancer treatment they had didn't w...
Summary: This study is designed to learn if enlicitide decanoate is safe and effective to treat children and adolescents with heterozygous familial hypercholesterolemia (HeFH) and high amounts of low-density lipoprotein cholesterol (LDL-C) in the blood. The goals of this study are to learn about the safety of enlicitide and if children tolerate it, what happens to enlicitide in a child's body over time, an...
Summary: This is a single-center, prospective, randomized, double-blind (pharmacotherapy), placebo-controlled, and comprehensive physiotherapy and nutritional intervention phase II clinical trial to determine the usefulness of nandrolone decanoate in a new indication (sarcopenia). Patients will be randomized 1:1 to receive nandrolone decanoate (50 mg intramuscular injection over four visits every 3 weeks) ...
Related Latest Advances
Brand Information
- Haloperidol 50 mg/mL (present as haloperidol decanoate) is a clear, yellow to light amber viscous liquid, free from visible foreign material, in a single-dose ampule
- Haloperidol 100 mg/mL (present as haloperidol decanoate) is a clear, yellow to light amber viscous liquid, free from visible foreign material, in a single-dose ampule
- Severe toxic central nervous system depression or comatose states from any cause.
- Known hypersensitivity to haloperidol or any components of HALDOL DECANOATE. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with haloperidol
- Parkinson's disease
- Dementia with Lewy bodies
- Sudden Death, Torsades de Pointes, and QTc Interval Prolongation
- Tachycardia and Hypotension
- Tardive Dyskinesia
- Neuroleptic Malignant Syndrome
- Seizures
- Hypersensitivity Reactions
- Leukopenia, Neutropenia, and Agranulocytosis
- Hyperprolactinemia
- Risk of Severe Neurotoxicity in Patients with Thyrotoxicosis
- 1 double-blind, active comparator-controlled trial with fluphenazine decanoate (Trial 1).
- 2 trials comparing HALDOL DECANOATE to oral haloperidol (Trials 2 and 3).
- 9 open-label trials.
- 1 dose-response trial.
- Cardiac Disorders:Tachycardia
- Endocrine Disorders:Hyperprolactinemia
- Eye Disorders:Vision blurred
- Gastrointestinal Disorders:Constipation, Dry mouth, Salivary hypersecretion
- General Disorders and Administration Site Conditions:Weight increased, Injection site reaction
- Musculoskeletal and Connective Tissue Disorders:Muscle rigidity
- Nervous System Disorders:Dyskinesia, Dystonia, Cogwheel rigidity, Hypertonia, Masked facies, Sedation, Somnolence
- Reproductive System Disorders:Erectile dysfunction
- Musculoskeletal and Connective Tissue Disorders:Torticollis, Trismus, Muscle twitching
- Nervous System Disorders:Neuroleptic malignant syndrome, Tardive dyskinesia, Bradykinesia, Hyperkinesia, Hypokinesia, Dizziness, Nystagmus
- Psychiatric Disorders:Loss of libido, Restlessness
- Reproductive System and Breast Disorders:Amenorrhea, Galactorrhea, Dysmenorrhea, Menorrhagia, Breast discomfort
- Skin and Subcutaneous Tissue Disorders:Acneiform skin reactions
- Vascular Disorders:Hypotension, Orthostatic hypotension
- Blood and Lymphatic System Disorders:Pancytopenia, Agranulocytosis, Thrombocytopenia, Leukopenia, Neutropenia
- Cardiac Disorders:Ventricular fibrillation, Torsade de pointes, Ventricular tachycardia, Extrasystoles, QTc interval prolongation
- Endocrine Disorders:Inappropriate antidiuretic hormone secretion
- Gastrointestinal Disorders:Vomiting, Nausea
- General Disorders and Administration Site Conditions:Sudden death, Face edema, Edema, Hyperthermia, Hypothermia, Injection site abscess, Weight decreased
- Hepatobiliary Disorders:Acute hepatic failure, Hepatitis, Cholestasis, Jaundice, Liver function test abnormal
- Immune System Disorders:Anaphylactic reaction, Hypersensitivity
- Metabolic and Nutritional Disorders:Hypoglycemia
- Musculoskeletal and Connective Tissue Disorders:Rhabdomyolysis
- Nervous System Disorders:Convulsion, Opisthotonus, Tardive dystonia
- Pregnancy, Puerperium and Perinatal Conditions:Neonatal drug withdrawal syndrome
- Psychiatric Disorders:Agitation, Confusional state, Depression, Insomnia
- Renal and Urinary Disorders:Urinary retention
- Reproductive System and Breast Disorders:Priapism, Gynecomastia
- Respiratory, Thoracic and Mediastinal Disorders:Laryngeal edema, Bronchospasm, Laryngospasm, Dyspnea
- Skin and Subcutaneous Tissue Disorders:Angioedema, Dermatitis exfoliative, Hypersensitivity vasculitis, Photosensitivity reaction, Urticaria, Pruritus, Rash, Hyperhidrosis
- Should hypotension occur and a vasopressor be required, epinephrine must not be used since HALDOL DECANOATE may block its vasopressor activity, and paradoxical further lowering of the blood pressure may occur. Instead, metaraminol, phenylephrine or norepinephrine should be used.
- In case of severe extrapyramidal reactions, antiparkinson drugs should be administered, and should be continued for several weeks, and then withdrawn gradually as extrapyramidal symptoms may emerge if discontinued abruptly.
- Monitor ECG and vital signs for signs of QTc interval prolongation or dysrhythmias and continue monitoring until the dysrhythmias resolve and the haloperidol-induced QTc interval prolongation resolves.
- Dialysis is not recommended in the treatment of overdose because it removes only very small amounts of haloperidol.
- 50 mg of haloperidol (present as 70.5 mg of haloperidol decanoate) in a sesame oil vehicle (0.85 g/mL), with 15 mg/mL benzyl alcohol as a preservative.
- 100 mg of haloperidol (present as 141 mg of haloperidol decanoate) in a sesame oil vehicle (0.85 g/mL), with 15 mg/mL benzyl alcohol as a preservative.
- haloperidol 50 mg/mL (present as haloperidol decanoate)
- haloperidol 100 mg/mL (present as haloperidol decanoate)
(HALOPERIDOL)
Decanoate 50
50 mg/mL*
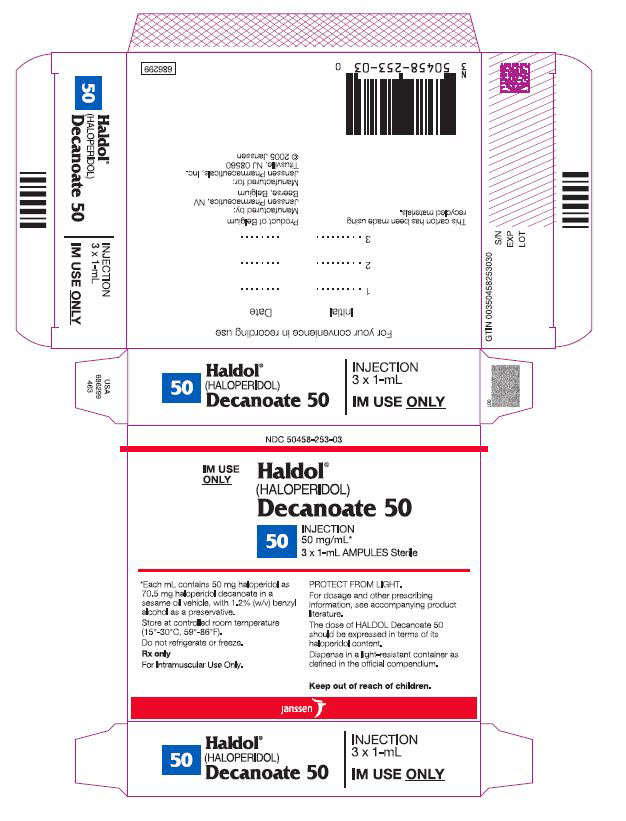
(HALOPERIDOL)
Decanoate 100
100 mg/mL*