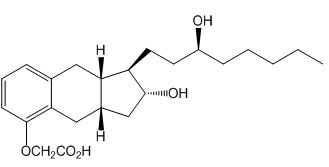
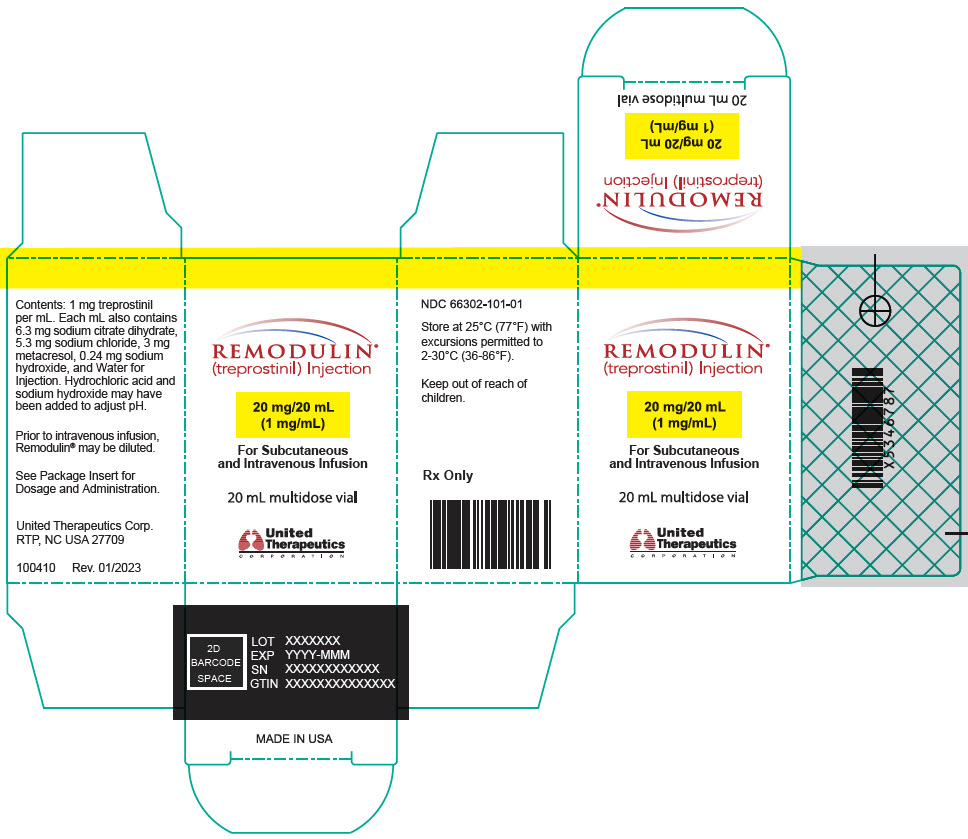
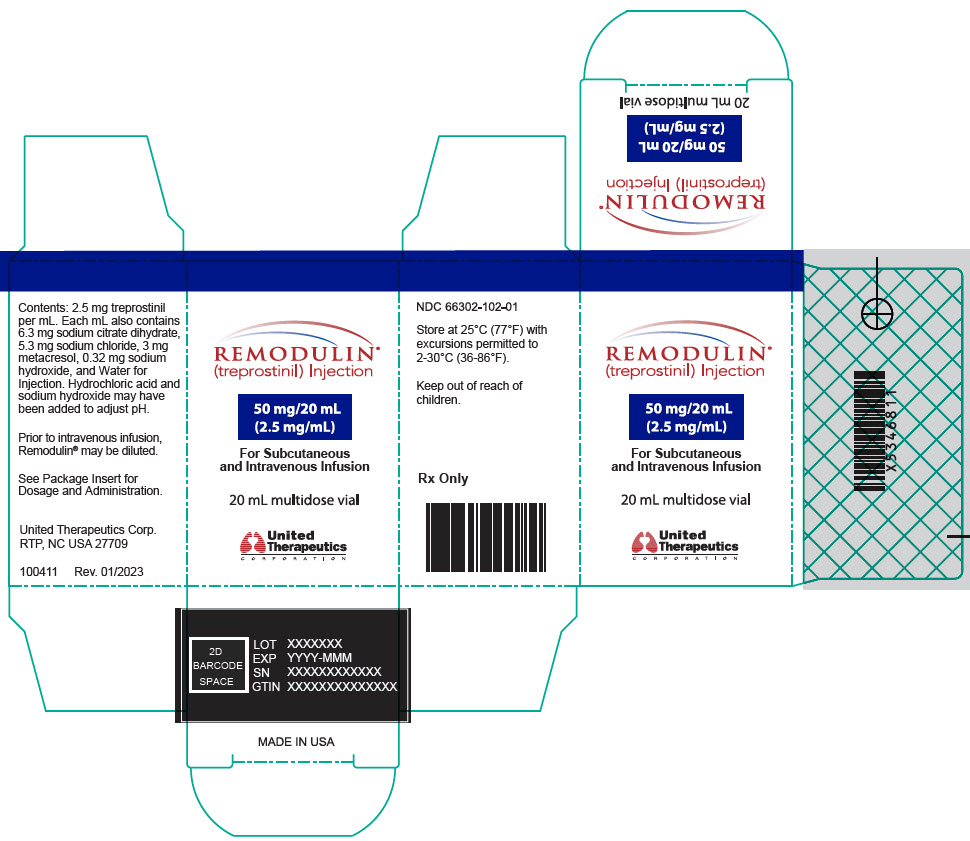
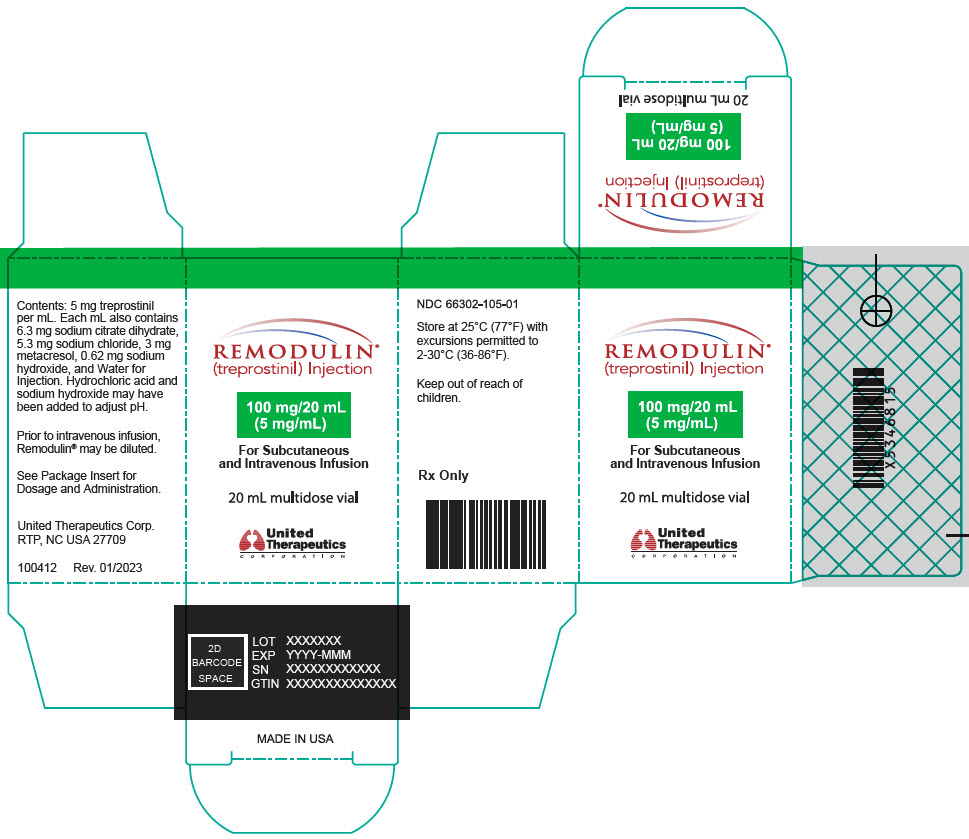
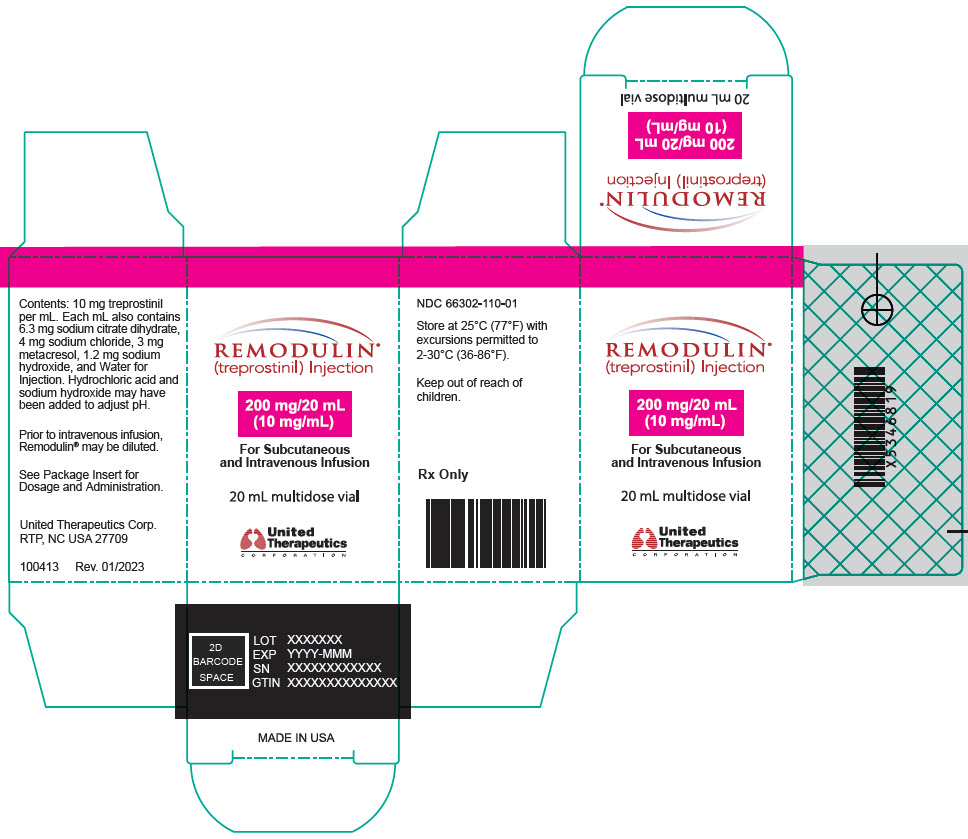
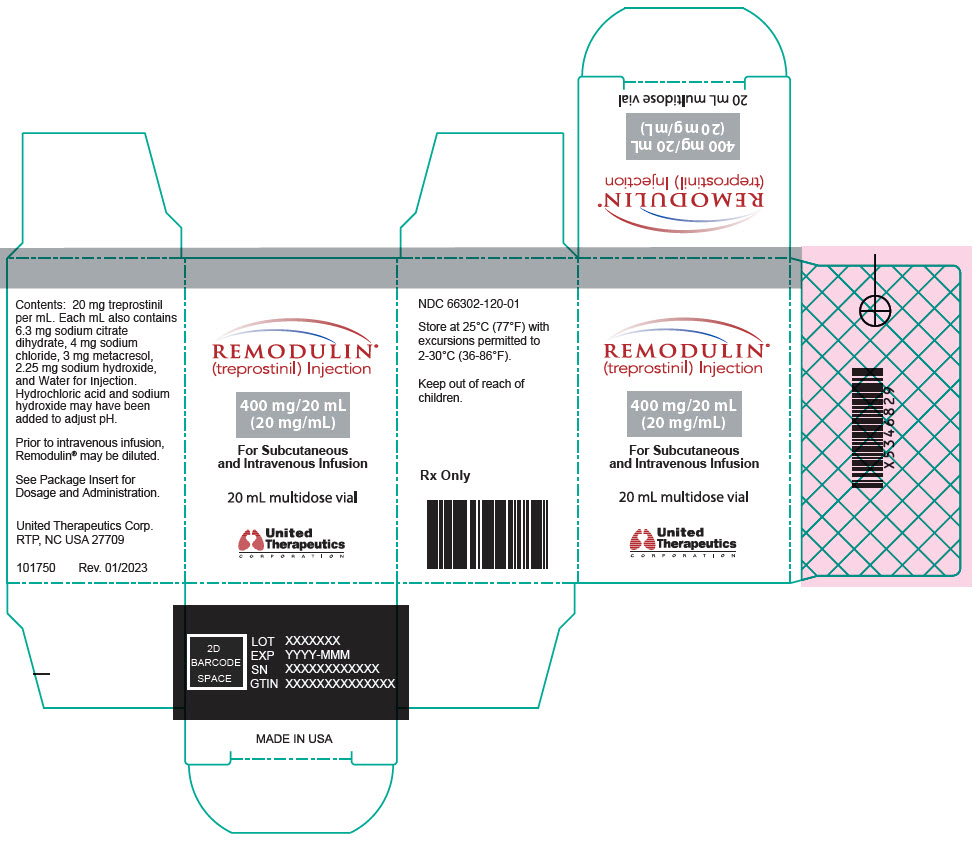
Aspart
What is Remodulin (Aspart)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The goal of this clinical trial is to evaluate the impact of inhaled Treprostinil on breathlessness and low exercise capacity in patients living with mild chronic obstructive pulmonary disease. Participants will be asked to: * Perform lung function and exercise tests * Have ultrasound of their heart * Have CT images of their lungs * Visit the lab on 5 different occasions (plus one visit to the CT ...
Summary: Study RIN-PF-305 is designed to evaluate the safety and efficacy of inhaled treprostinil in subjects with progressive pulmonary fibrosis (PPF) over a 52-week period.
Summary: This study compares insulin icodec, an insulin taken once a week to insulin glargine, an insulin taken once a day. The study medicine will be investigated in participants with type 1 diabetes. The study will look at how well insulin icodec taken weekly controls blood sugar compared to insulin glargine taken daily. The study will last for about 8.5 months.
Related Latest Advances
Brand Information